Figure 1.
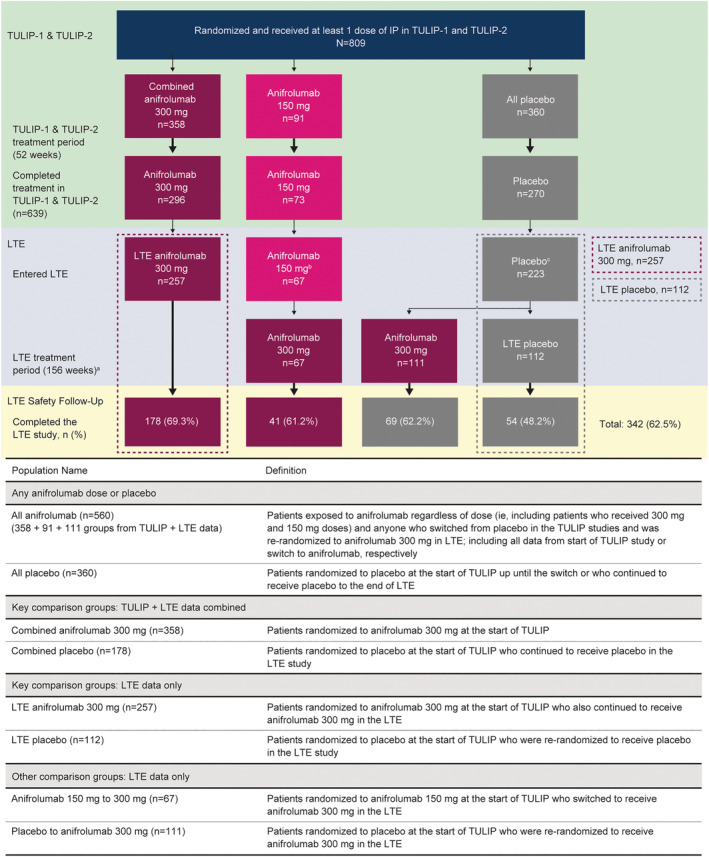
Patients included in the TULIP‐1 or TULIP‐2 trials and LTE study: treatment randomization and treatment group definitions. aThere was an 8‐week safety follow‐up period. bPatients randomized to receive anifrolumab 150 mg were all in TULIP‐1. cPatients were re‐randomized to receive anifrolumab 300 mg or placebo for the LTE study. TULIP‐1 = Treatment of Uncontrolled Lupus via the Interferon Pathway 1; IP = investigational product; LTE = long‐term extension.
