Abstract
Prone positioning is an evidence-based treatment for patients with moderate-to-severe acute respiratory distress syndrome. Lung recruitment has been proposed as one of the mechanisms by which prone positioning reduces mortality in this group of patients. Recruitment-to-inflation ratio (R/I) is a method to measure potential for lung recruitment induced by a change in positive end-expiratory pressure (PEEP) on the ventilator. The association between R/I and potential for lung recruitment in supine and prone position has not been studied with computed tomography (CT) scan imaging. In this secondary analysis, we sought to investigate the correlation between R/I measured in supine and prone position with CT and the potential for lung recruitment as measured by CT scan. Among 23 patients, the median R/I did not significantly change from supine (1.9 IQR 1.6–2.6) to prone position (1.7 IQR 1.3–2.8) (paired t test p = 0.051) but the individual changes correlated with the different response to PEEP. In supine and in prone position, R/I significantly correlated with the proportion of lung tissue recruitment induced by the change of PEEP. Lung tissue recruitment induced by a change of PEEP from 5 to 15 cmH2O was 16% (IQR 11–24%) in supine and 14.3% (IQR 8.4–22.6%) in prone position, as measured by CT scan analysis (paired t test p = 0.56). In this analysis, PEEP-induced recruitability as measured by R/I correlated with PEEP-induced lung recruitment as measured by CT scan, and could help to readjust PEEP in prone position.
Keywords: Acute respiratory distress syndrome, Prone positioning, Recruitment-to-inflation ratio, Lung recruitment
Introduction
Prone positioning is an established treatment for patients with moderate-to-severe acute respiratory distress syndrome (ARDS) resulting in a significant survival benefit [1, 2]. Several studies suggest that this benefit derives from reduction of ventilator-induced lung injury (VILI), rather than improvement in gas exchange [3]. Lung recruitment has been identified as one of the mechanisms by which prone positioning reduces VILI [4].
Recently, Chen et al. described the recruitment-to-inflation ratio (R/I), a method to measure potential for lung recruitment induced by a change in positive end expiratory pressure (PEEP) [5]. Interestingly, Cour et al. showed that the change of R/I from supine to prone position in patients with COVID-19 related ARDS significantly correlated to changes of the compliance of the respiratory system. In high recruiters (R/I above median), the improvement of compliance, oxygenation and ventilatory ratio in prone position compared to the supine baseline measurement was associated with a reduction of R/I, suggesting less recruitability in prone position due to true lung recruitment. In low recruiters (R/I below median), only oxygenation improved in prone compared to the supine position, without significant changes in R/I [6]. Nonetheless, the association between R/I and potential for lung recruitment in supine and prone position has never been explored with computed tomography (CT) scan imaging.
Cornejo et al. studied in 24 patients with ARDS the potential for lung recruitment in supine and prone position by analysis of CT scan imaging performed at PEEP of 5, 15 and 45 cmH2O. CT scans were performed within 30 min of position change [4]. The results demonstrated that prone position significantly increases lung recruitment and reduces high PEEP-induced alveolar hyperinflation. In this study, the measurements of the respiratory system compliance and the CT scan-based lung volume at PEEP of 5 and 15 cmH2O allows to retrospectively calculate a CT scan-based R/I. This is calculated using the original R/I equation and replacing the bedside measurement of the difference of end expiratory lung volume between PEEP-high and PEEP-low with the difference of gas volumes measured by CT scan between PEEP of 15 and 5 cmH2O, according to the following equation: [5, 7]
R/I = Crec/Crs at PEEP5cmH2O
Crec = DeltaVrec/(PEEPhigh − PEEPlow)
DeltaVrec = Delta end expiratory lung volume between PEEPhigh and PEEPlow − Predicted Delta end expiratory lung volume
Delta end expiratory lung volume between PEEPhigh and PEEPlow = CT gas volume PEEP15cmH2O − CT gas volumePEEP5cmH2O
Predicted Delta end expiratory lung volume = Crs at PEEP5cmH2O × (PEEP15cmH2O − PEEP5cmH2O) = Crs at PEEP5cmH2O × 10 cmH2O
PEEPhigh − PEEPlow = PEEP15cmH2O − PEEP5cmH2O = 10 cmH2O
Crs at PEEP5cmH2O = compliance of the respiratory system at PEEP of 5 cmH2O
Therefore, we performed a secondary analysis of the data from the study by Cornejo et al. to investigate whether R/I measured in in supine and prone position correlates with potential for lung recruitment, as measured by CT scan. [4]
Methods
We calculated the CT scan-based R/I for each patient of the study in supine and prone position as described above. The distribution of the variables of interest is expressed as median and interquartile range (IQR).
To calculate PEEP-induced lung recruitment by CT scan we used the method described by Chiumello et al. measuring the proportion of total lung weight of noninflated (NAT) and poorly inflated (PAT) tissue recruited when changing PEEP from 5 to 15 cmH2O, according to the following equation: [7]
To evaluate the relationship between R/I and lung recruitment as measured by CT scan we used Rho Spearman correlation. R2 is shown as a measure of model fit.
Results
Twenty-three out of twenty-four subjects in the original study were included in this reanalysis. One subject was excluded as a CT scan was missing and precluded our reanalysis.
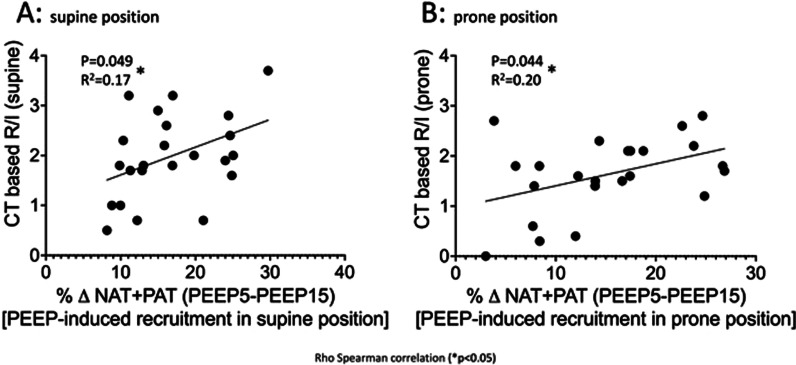
The median R/I did not significantly change from supine (1.9 IQR 1.6–2.6) to prone position (1.7 IQR 1.3–2.8) (paired t test p = 0.051). The proportion of lung tissue recruitment induced by the change of PEEP from 5 to 15 cmH2O was 16% (IQR 11–24%) in supine and 14.3% (IQR 8.4–22.6%) in prone position, as measured by CT scan analysis (paired t test p = 0.56). In supine (Fig. 1A) and in prone (Fig. 1B) position, the R/I significantly correlated with the proportion of lung tissue recruitment induced by the change of PEEP.
Fig. 1.
Association between R/I and lung recruitment as measured by CT scan analysis, in supine (A) and prone (B) position. NAT = Non aerated tissue; PAT = poorly aerated tissue; PEEP = positive end-expiratory pressure
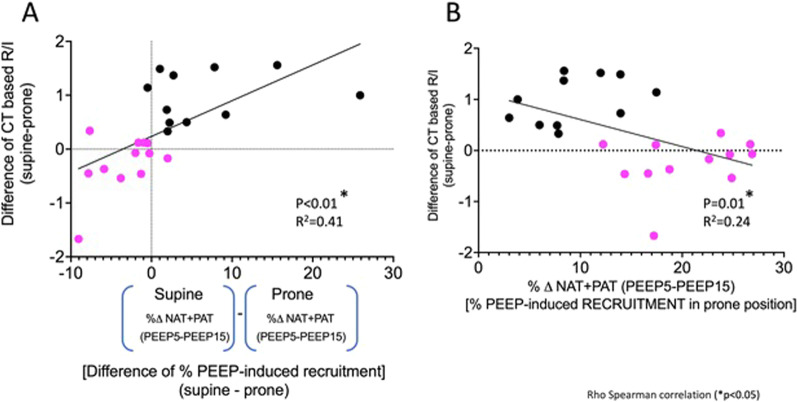
We used the ΔR/I (R/Isupine − R/Iprone) to express the individual change in recruitability between prone and supine position. This value significantly correlated with the difference in PEEP-induced lung recruitment in prone compared to supine position, as measured by CT scan (supinePEEP-induced lung tissue recruitment% − pronePEEP-induced lung tissue recruitment%) (Fig. 2A). When these two values were plotted, patients were mainly distributed in two quadrants. In the left lower quadrant, patients (pink dots) have a negative ΔR/I, indicating that recruitability is higher in prone compared to supine position. In these patients, PEEP-induced recruitment, as measured by CT scan, was also higher in prone than supine position. In the right upper quadrant, patients (black dots) have a positive ΔR/I, indicating that PEEP-induced recruitability is lower in prone compared to supine position. In these patients, PEEP-induced recruitment, as measured by CT scan, was also higher in supine than prone position, suggesting that the reduced recruitability in prone position is the result of a true lung recruitment. Indeed, the ΔR/I inversely correlated with the portion of tissue lung recruited from PEEP 5 to 15 cmH2O in prone position (Fig. 2B); therefore, the higher the ΔR/I, the lower is the recruitment induced by PEEP in prone position.
Fig. 2.
A Association between ΔR/I (difference of R/Isupine − R/Iprone) and the difference of the percentage of PEEP-induced recruitment between supine and prone position, as measured by CT scan. Patients were mainly distributed in two quadrants. In the left lower quadrant, pink dots represent the group of patients in which recruitability is higher in prone compared to supine position (negative ΔR/I). In these patients, PEEP-induced recruitment, as measured by CT scan, was also higher in prone than supine position. In the right upper quadrant, black dots represent the group of patients with a positive ΔR/I, indicating that PEEP-induced recruitability is lower in prone compared to supine position. B: Association between ΔR/I and PEEP-induced recruitment in prone position. The group of patients color-coded in panel A show in panel B lower recruitment induced by PEEP in prone position, suggesting that lung recruitment has already occurred due to the turn in prone position
Discussion
Our study demonstrated that PEEP-induced recruitability as measured by R/I significantly correlates with PEEP-induced lung recruitment as measured by CT scan, in both prone and supine position. The correlation is stronger in prone position compared to supine position, which could potentially be explained by the lower pleural pressure gradient in prone position, resulting in more homogeneous distribution of aeration, and by undetected higher airway opening pressure in supine position, which results in underestimation of the respiratory system compliance. Our results show that the calculation of the ΔR/I between supine and prone position may enable the identification of patients with different recruitment response to PEEP when in prone position. Patients with negative ΔR/I have higher PEEP-induced recruitment while in prone position and hence may benefit from a high PEEP strategy. Conversely, patients with positive ΔR/I, in whom lung recruitment has already occurred due to the turn in prone position, may require low levels of PEEP.
Our study has important limitations. First, it is a secondary analysis of a study with a small sample size, and we could only reanalyze twenty-three out of twenty-four subjects on the original study. Second, patients were recruited only in one centre. Third, for the calculation of R/I lung volumes was not measured from the ventilator at the bedside but calculated from analysis of CT scan imaging in static conditions, which may overestimate gas volumes [8].
In conclusion, measurement of R/I in supine and prone position correlates with CT scan-assessed lung recruitability and may be instrumental in investigating the heterogeneity of treatment effect across individuals receiving prone position.
Acknowledgements
None.
Abbreviations
- VILI
Ventilator-induced lung injury
- ARDS
Acute respiratory distress syndrome
- R/I
Recruitment-to-inflation ratio
- PEEP
Positive end-expiratory pressure
- CT
Computed tomography
Author contributions
LDS, RC and MT contributed to Conception and design. LDS, RC and MT contributed to Analysis and interpretation. LDS, RC and MT contributed to Drafting of the manuscript. LDS, MT, LC, LB, DA, RG, JCD and RC contributed to Revision of the manuscript. All authors agree with the final version. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This is non-interventional data-based research. There is no processing of indirectly identifiable data, or chaining with data from other sources, or long-term patient follow-up for this research.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorenzo Del Sorbo, Manuel Tisminetzky and Rodrigo Cornejo contributed equally to this manuscript.
Contributor Information
Lorenzo Del Sorbo, Email: lorenzo.delsorbo@uhn.ca.
Rodrigo Cornejo, Email: racornej@gmail.com.
References
- 1.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. New Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/nejmoa1214103. [DOI] [PubMed] [Google Scholar]
- 2.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S288. doi: 10.1513/annalsats.201704-343ot. [DOI] [PubMed] [Google Scholar]
- 3.Albert RK, Keniston A, Baboi L, Ayzac L, Guérin C, Investigators P. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care. 2014;189(4):494–496. doi: 10.1164/rccm.201311-2056le. [DOI] [PubMed] [Google Scholar]
- 4.Cornejo RA, Díaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care. 2013;188(4):440–448. doi: 10.1164/rccm.201207-1279oc. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Del Sorbo L, Grieco DL, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care. 2020;201(2):178–187. doi: 10.1164/rccm.201902-0334oc. [DOI] [PubMed] [Google Scholar]
- 6.Cour M, Bussy D, Stevic N, Argaud L, Guérin C. Differential effects of prone position in COVID-19-related ARDS in low and high recruiters. Intens Care Med. 2021;47:1044–1046. doi: 10.1007/s00134-021-06466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiumello D, Marino A, Brioni M, et al. Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome. What is the relationship? Am J Respir Crit Care. 2016;193(11):1254–1263. doi: 10.1164/rccm.201507-1413oc. [DOI] [PubMed] [Google Scholar]
- 8.Chiumello D, Cressoni M, Chierichetti M, et al. Nitrogen washout/washin, helium dilution and computed tomography in the assessment of end expiratory lung volume. Crit Care. 2008;12(6):R150. doi: 10.1186/cc7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.