Figure 22.
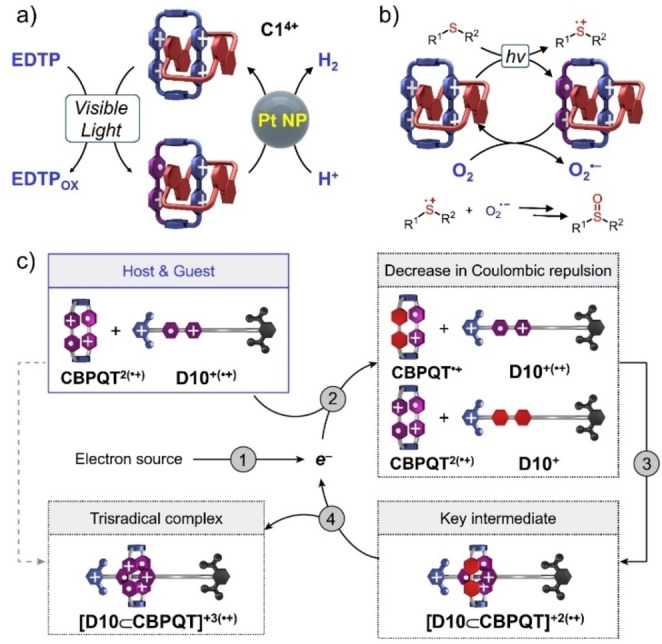
a) Schematic representation of the visible‐light‐induced electron‐transfer process in the donor–acceptor [2]catenane C1 4+ in the catalytic generation of H2 gas in the presence of platinum nanoparticles as co‐catalysts and EDTP as a sacrificial reductant. b) Schematic representation of the visible‐light‐induced electron‐transfer process in C1 4+ in the catalytic oxidation of organic sulfides in the presence of O2. c) Schematic representation of the mechanism for an electron‐catalyzed molecular recognition process. The direct (gray dashed arrow) formation of a [D9⊂CBPQT] +3(⋅+) trisradical complex from CBPQT 2⋅+ and D9 +(⋅+) is kinetically forbidden. The catalytic (black solid arrows) complexation comprises four steps, including one initiating step and three propagating steps. Step 1—the injection of an electron. Step 2—a single‐electron reduction of either one of the V⋅+ units in CBPQT 2⋅+ or the V⋅+ unit in D9 +(⋅+). Step 3—the rapid formation of a [D9⊂CBPQT] +2(⋅+) bisradical complex, favored by a decrease in the Coulombic repulsion between the host and guest molecules. Step 4—the oxidation of the [D9⊂CBPQT] +2(⋅+) bisradical complex to the trisradical complex, while releasing an electron to close the catalytic cycle. Steps 1/2/3/4 are portrayed inside grey full moons.
