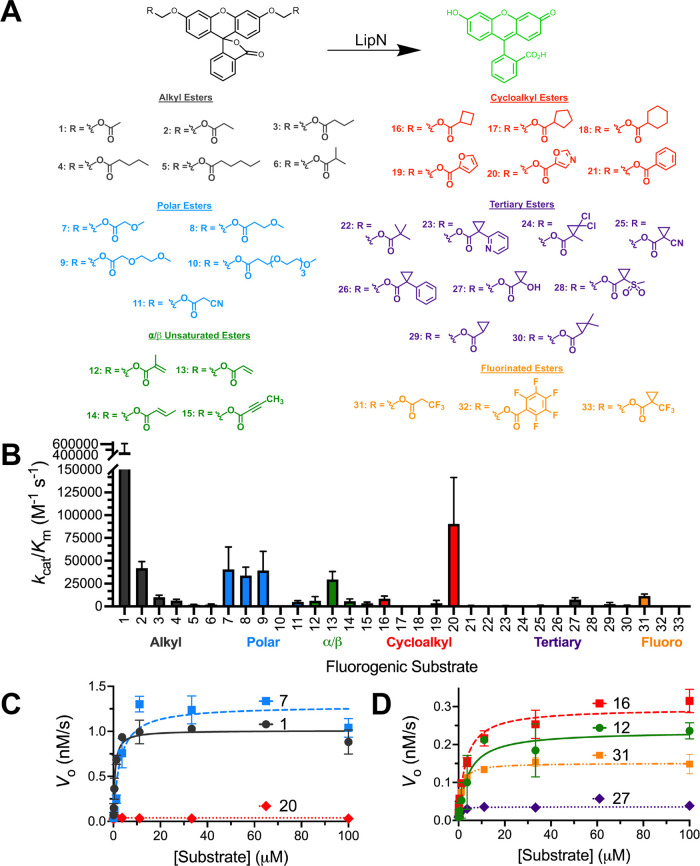
Figure 1.
Steady-state kinetic analysis of MmLipN. (A) Activation of fluorogenic ester substrates by MmLipN. Each of the substrates is composed of diacyloxymethyl ether fluorescein with varying R-groups. The differing R-groups have been organized into classes and colored based on chemical functionality. All of the substrates were synthesized as described previously.38,43 Substrates 6, 10–11, 14–15, 20, 23–30, and 33 were newly synthesized for this characterization of MmLipN. Detailed synthetic characterization is provided in the Supporting Information. (B) Global comparison of the catalytic specificity (kcat/KM) of MmLipN against each of the 33 substrates with ester classes labeled. Catalytic efficiency values (kcat/Km) are given ± SD based on at least three independent kinetic replicates. Detailed kinetic results for each substrate are provided in Table S1. (C, D) Kinetic activity of MmLipN against high-activity ester substrates 1 (black circles), 7 (blue squares), and 20 (red diamonds) and low-activity ester substrates 12 (green circles), 16 (red squares), 27 (purple diamonds), and 31 (orange squares). Low- and high-activity substrates display classic Michaelis–Menten kinetics with MmLipN and activity above background hydrolysis rates. Traces colored based on ester classification from (A, B). All measurements were completed in at least triplicate and shown ± SD.