Table 3. Few Apparent Thermodynamic Properties of RST in the {PEG400 (1) + Water (2)} Cosolvent Mixture at 298.15 K and Pressure (p) = 0.1 MPad.
| w1a | f1a | x1a | x3c | δ1+2b | ΔsolnG°/kJ·mol–1c |
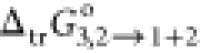 /kJ·mol–1c /kJ·mol–1c
|
|---|---|---|---|---|---|---|
| 0.00 | 0.0 | 0.0 | 2.15 × 10–6 | 47.8 | 32.36 | 0.00 |
| 0.10 | 0.0898 | 0.0050 | 5.63 × 10–6 | 45.66 | 29.97 | –2.39 |
| 0.20 | 0.1817 | 0.0111 | 8.92 × 10–6 | 43.48 | 28.83 | –3.53 |
| 0.30 | 0.2757 | 0.0189 | 1.97 × 10–5 | 41.24 | 26.86 | –5.49 |
| 0.40 | 0.3719 | 0.0292 | 5.27 × 10–5 | 38.95 | 24.42 | –7.93 |
| 0.50 | 0.4704 | 0.0431 | 6.59 × 10–5 | 36.60 | 23.87 | –8.49 |
| 0.60 | 0.5713 | 0.0633 | 7.91 × 10–5 | 34.20 | 23.42 | –8.94 |
| 0.70 | 0.6745 | 0.0951 | 9.75 × 10–5 | 31.75 | 22.90 | –9.46 |
| 0.80 | 0.7804 | 0.1527 | 1.39 × 10–4 | 29.23 | 22.02 | –10.34 |
| 0.90 | 0.8888 | 0.2885 | 1.97 × 10–4 | 26.65 | 21.15 | –11.20 |
| 1.00 | 1.0 | 1.0 | 6.70 × 10–4 | 24.0 | 18.12 | –14.24 |
w1, f1, and x1 are the mass, volume, and mole fractions of PEG 400 (1) in the {PEG 400 (1) + water (2)} mixtures free of RST (3). Volume fractions of PEG400 were calculated assuming additive behavior from density values reported by Rodriguez et al.29
δ1+2 is the Hildebrand solubility parameter of {PEG 400 (1) + water (2)} mixtures free of RST at 298.15 K.
x3 is the mole fraction solubility of RST.
Standard uncertainties u are u (T) = 0.02 K for temperature and u (p) = 0.001 MPa for pressure with 0.95% level of confidence. x3 = mole fraction solubility at standard uncertainties u of u (T) = 0.02 K for temperature and u (p) = 0.001 MPa for pressure with 0.95% level of confidence.
