FIGURE 1.
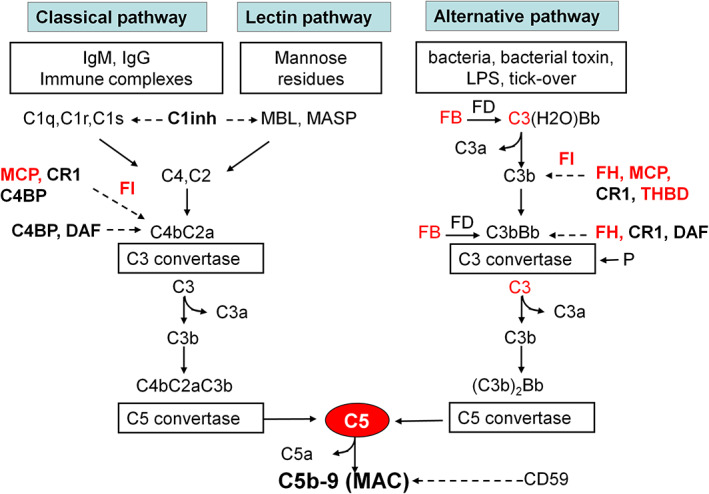
The complement cascade. Schematic overview of the complement cascade, illustrating the three activation pathways (classical, lectin, and alternative) with the C3 convertase complexes of the classical, lectin, and the alternative pathway and the common terminal pathway that leads to C5 cleavage and the formation of the anaphylatoxin C5a and of the membrane attack complex, composed of C5b, C6, C7, C8, and many copies of C9. The classical pathway is triggered by the binding of C1q to antibody‐antigen complexes. The lectin pathway is similar to the classical pathway but is activated by the binding of mannose‐binding lectin (MBL) to mannose residues, which activates mannose‐binding lectin serine peptidase (MASP) proteins. In contrast, the alternative pathway is continuously activated in plasma by low‐grade hydrolysis of C3 (C3H2O, tick‐over). The latter binds factor B, to form a C3(H2O)B complex. Factor D cleaves factor B to form the alternative pathway initiation C3 convertase that cleaves C3 to C3b. The activation is then amplified by the covalent binding of a small amount of C3b to hydroxyl groups on cell surface carbohydrates and proteins of target cells, such as bacterial cells. This C3b binds factor B, to form the amplification loop C3 convertase C3bBb. C3b also binds to the C3 convertase, forming the C5 convertase enzyme C3b2Bb. The alternative pathway is highly regulated to prevent non‐specific damage to host cells and limit the deposition of complement on the surface of pathogens. This fine regulation occurs through a number of membrane‐anchored and fluid‐phase regulators. Bold text denotes complement‐regulatory molecules; red text denotes proteins with genetic defects that have been associated with aHUS and/or IC‐MPGN/C3G. Abbreviations and definitions: C1inh, C1 inhibitor (inactivates C1r and C1s, MASP‐1 and MASP‐2); FB, complement factor B; FD, complement factor D; FH, complement factor H (binds C3b, exerts cofactor activity for FI‐mediated C3b cleavage, prevents the formation of the alternative pathway C3 convertase, and destabilizes (decay accelerating activity) the alternative pathway C3 and C5 convertases); FI, complement factor I (degrades C3b and C4b, aided by cofactors); C4BP, C4b‐binding protein (binds to C4b and has decay accelerating activity for the classical pathway C3 convertase and cofactor activity for FI‐mediated C4b cleavage); CD59, protectin (with vitronectin and clusterin, prevents C5b‐9 formation); CR1, complement receptor 1 (has decay accelerating activity as well as cofactor activity for FI‐mediated C3b and C4b cleavage); DAF, decay accelerating factor (has decay accelerating activity on C3/C5 convertases of the classical and alternative pathways); MCP, membrane cofactor protein (exerts cofactor activity for FI‐mediated C3b cleavage); P, properdin (the only positive regulator in the complement system, it stabilizes the alternative pathway C3 convertase); THBD, thrombomodulin (increases FH cofactor activity, activates procarboxypeptidase B‐mediated C3a and C5a inactivation).
