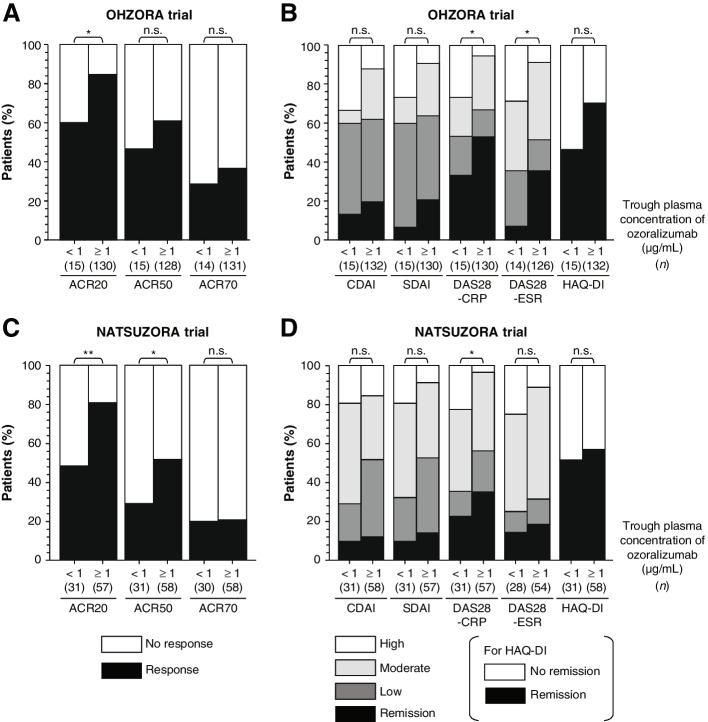
Fig. 5.
Efficacy stratified by trough concentration of ozoralizumab (1 μg/mL) at week 16 in the ozoralizumab 30 mg group in the OHZORA trial (A, B) and the NATSUZORA trial (C, D). No remission, HAQ-DI > 0.5; High, CDAI > 22, SDAI > 26, DAS28 > 5.1; moderate, 10 < CDAI ≤ 22, 11 < SDAI ≤ 26, 3.2 ≤ DAS28 ≤ 5.1; low, 2.8 < CDAI ≤ 10, 3.3 < SDAI ≤ 11, 2.6 ≤ DAS28 < 3.2; remission, CDAI ≤ 2.8, SDAI ≤ 3.3, DAS28 < 2.6, HAQ-DI ≤ 0.5. Fisher’s exact test, *p < 0.05, **p < 0.01. ACR20/50/70 ≥ 20%/50%/70% improvement according to the American College of Rheumatology criteria, CDAI Clinical Disease Activity Index, CRP C-reactive protein, DAS28-CRP Disease Activity Score in 28 joints based on CRP, DAS28-ESR Disease Activity Score in 28 joints based on ESR, ESR erythrocyte sedimentation rate, HAQ-DI Health Assessment Questionnaire-Disability Index, n.s. not significant, SDAI Simplified Disease Activity Index