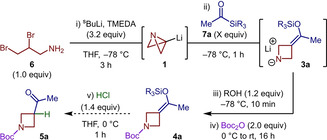
Table 1.
Optimization studies.
|
| ||||
|---|---|---|---|---|
|
Entry |
SiR3 |
7 a; X equiv |
ROH |
4 a; % Yield[a] |
|
1 |
TMS (7 a) |
1.2 |
– |
19–45[b] |
|
2 |
TMS (7 a) |
1.2 |
MeOH |
5 |
|
3 |
TMS (7 a) |
1.2 |
iPrOH |
34 |
|
4 |
TMS (7 a) |
1.2 |
tBuOH |
50 |
|
5 |
TMS (7 a) |
2.0 |
tBuOH |
25 |
|
6 |
TMS (7 a) |
1.0 |
tBuOH |
57 |
|
7 |
TMS (7 a) |
0.7 |
tBuOH |
64 (63)[c] |
|
8 |
TES (7a′) |
0.7 |
tBuOH |
50[d] |
|
9 |
TBS (7 a′′) |
0.7 |
tBuOH |
43[d] |
Reactions performed with 0.46 mmol of 6. [a] 1H NMR yield of 4 a. [b] Range of yields across 5 experiments, no detectable byproducts observed. [c] Yield of isolated 5 a (relative to limiting reagent 7) after silyl enol ether hydrolysis. [d] Yield of isolated 4 a relative to limiting reagent 7.