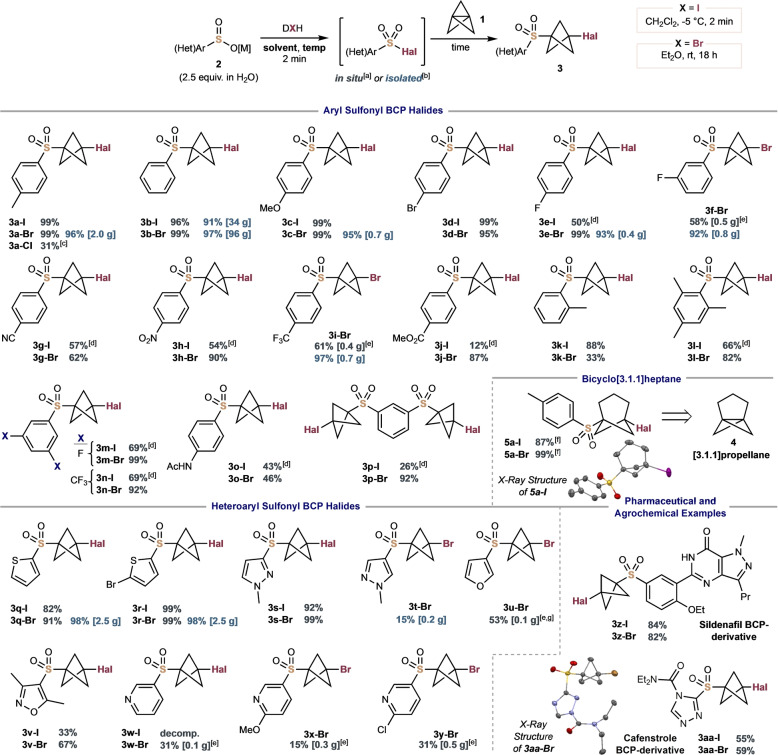
Figure 2.
Scope of halosulfonylation of [1.1.1] and [3.1.1]propellane with aryl and heteroaryl sulfinates, [M]=Na or Li. [a] Yields in grey represent sulfonyl halides prepared in situ from 2.5 equiv of 2 (1.0 M in H2O) and 1.0 equiv of DIH/DBH/DCH, then 1.0 equiv of 1 (0.70–0.75 M in Et2O) according to the title scheme on a 0.2 mmol scale. [b] Yields in blue represent use of 1.0 equiv of isolated sulfonyl halide and 1.3 equiv of 1 in Et2O at rt for 15 h. [c] DCH instead of DBH, 10 mol % Et3B, 2 h, rt. [d] 2 (1.0 M in DMF) was iodinated at −40 °C; after addition of 1, stirred at −40 °C for 20 min, then rt, 10 min. [e] 1.0 equiv of 2 was brominated using 1.0 equiv of NBS in MeCN at rt for 30 min; then 1.5 equiv of 1, 15 h. [f] 1.0 equiv of [3.1.1]propellane 4 (0.23 M in Et2O) instead of 1. [g] 0.95 equiv of PPh3⋅Br2, 1.0 equiv of 2 and 1.5 equiv of 1 in MeCN for 15 h at rt. Structure of 5 a‐I and 3 aa‐Br from X‐ray diffraction studies, displacement ellipsoids are drawn at 50 % probability and hydrogen atoms are omitted for clarity. [14]