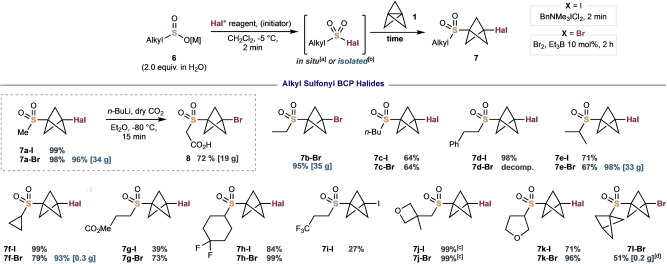
Figure 3.
Scope of alkyl sulfonyl BCP halides. [a] Yields in grey represent sulfonyl halides prepared in situ from 2.0 equiv of 6 (1.0 M in H2O, [M]=Na or Li) and 1.0 equiv of 1 (0.70–0.75 M in Et2O) according to the title scheme on a 0.1 mmol scale. BCP iodides: 1.4 equiv of BnNMe3ICl2 was used. BCP bromides: 1.8 equiv of Br2 with 10 mol % of Et3B was used. [b] Yields in blue represent use of 1.0 equiv of isolated sulfonyl halide and 1.3 equiv of 1, in Et2O for 15 h at rt, without initiator. [c] 0.5 μmol scale. [d] 0.95 equiv of PPh3⋅Br2, 1.0 equiv of 6 and 1.5 equiv of 1 in MeCN at rt for 15 h.