Scheme 1.
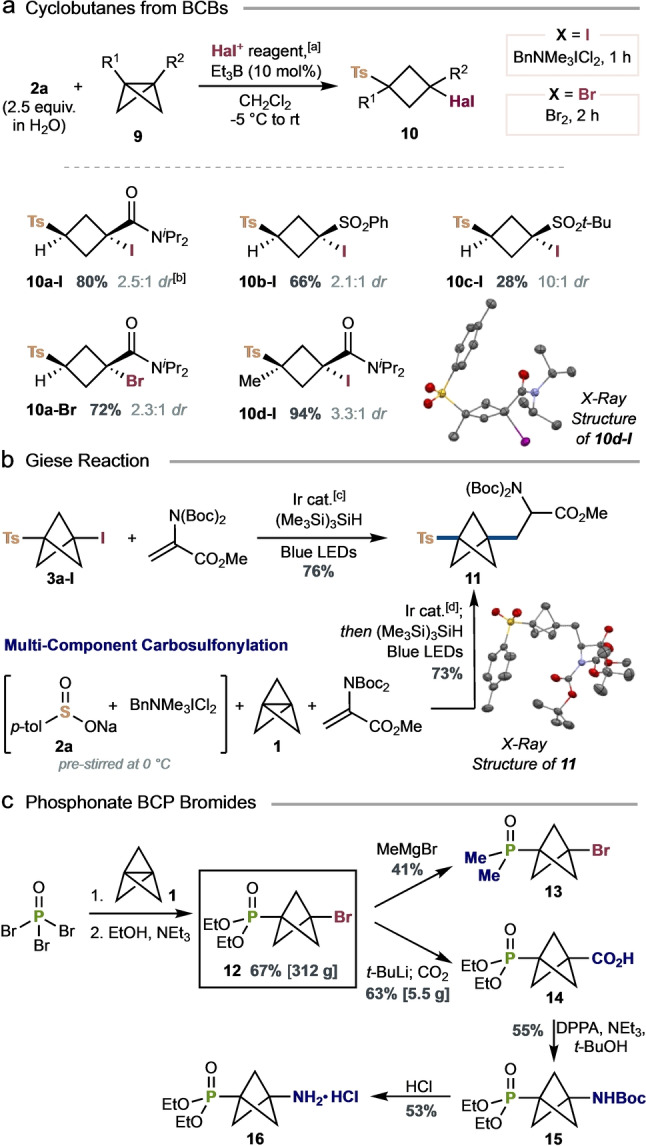
a) Addition of sulfonyl halides to BCBs. b) Giese reaction of a sulfonyl BCP iodide. c) Synthesis and functionalization of phosphonate BCP bromides (see the Supporting Information for detailed reaction conditions). [a] Reactions carried out on a 0.1 mmol scale according to the title scheme, using 2.5 equiv of 2 a (1.0 M in H2O) and either 1.4 equiv of BnNMe3ICl2 or 1.8 equiv of Br2, stirred for 2 min before addition of 1.0 equiv of BCB 9 (0.1 M in CH2Cl2) and 10 mol % Et3B. [b] The major diastereoisomer of 10 b‐I was assigned by NOESY correlations; other adducts were assigned by comparison of 1H NMR spectra. [c] 2.5 mol % Ir[(dF(CF3)ppy)2(dtbbpy)]PF6, 2.0 equiv of Na2CO3, 2.0 equiv of (Me3Si)3SiH, 6.0 equiv, of dehydroalanine, MeOH/H2O (1 : 1), blue LEDs, rt, 18 h. [d] 2.5 equiv of 2 a (1.0 M in H2O) and 1.4 equiv of BnNMe3ICl2 in CH2Cl2 were pre‐stirred at 0 °C for 2 min, then added to a vial containing reagents as in [c] and 1.0 equiv of 1; (Me3Si)3SiH was added last. Structure of 10 d‐I and 11 from X‐ray diffraction studies, displacement ellipsoids are drawn at 50 % probability, hydrogen atoms and disordered solvent (11 only) are omitted for clarity. [14] Boc=tert‐butoxycarbonyl. DPPA=diphenylphosphoryl azide.
