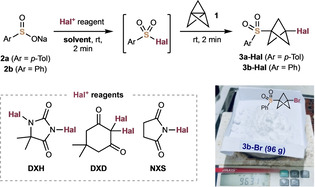
Table 1.
Optimization of halosulfonylation of 1.[a]
|
| ||||
|---|---|---|---|---|
|
|
|
|
|
|
|
Entry |
Substrate |
Hal+ |
Solvent |
Yield 3 a or 3 b [%][b] |
|
1 |
2 a |
NIS |
THF |
41 |
|
2 |
2 a |
ICl |
THF |
49 |
|
3 |
2 a |
DIH |
THF |
78 |
|
4 |
2 a |
DID |
THF |
31 |
|
5 |
2 a |
I2 |
THF |
7[c] |
|
6 |
2 a |
DIH |
Et2O |
quant. |
|
7 |
2 a |
DIH |
H2O |
99 |
|
8 |
2 a |
DIH |
CH2Cl2 |
quant.[d] |
|
9[e] |
2 a |
DIH |
Et2O |
nr |
|
10 |
2 a |
DBH |
Et2O |
9 |
|
11 |
2 a |
DBH |
Et2O |
quant.[d,f] |
|
12 |
2 a |
NBS |
Et2O |
50[f] |
|
13 |
2 b |
I2 |
Et2O |
91[d,g] |
|
14 |
2 b |
Br2 |
Et2O |
97[d,g] |
[a] Optimization carried out on 0.15 mmol scale, with 2.5 equiv sulfinate (1.0 M in H2O), 1.0 equiv of halogenating agent and 1.0 equiv of 1 (0.75 M in Et2O). [b] 1H NMR yields calculated with mesitylene as internal standard. [c] 18 % di‐iodo BCP observed. [d] Isolated yield. [e] Reaction run in the dark. [f] 18 h reaction time after addition of 1. [g] 1.0 equiv of pre‐isolated sulfonyl halide and 1.3 equiv of 1 were reacted in Et2O for 15 h at rt. X/Hal=Halogen. DXH=dihalohydantoin. DXD=dihalodimedone. NXS=N‐halosuccinimide p‐Tol=para‐tolyl. nr = no reaction.