Figure 1.
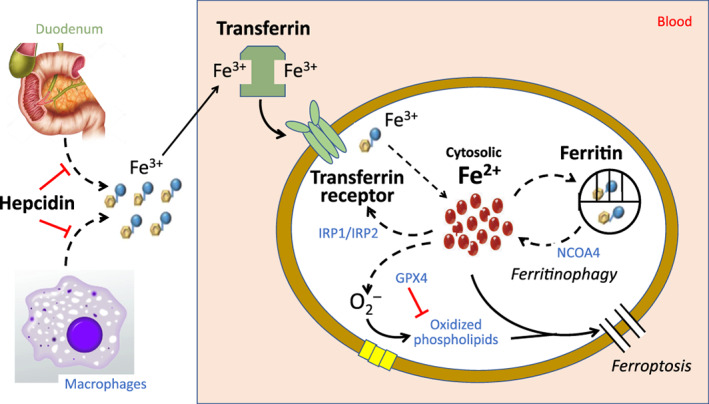
Mechanisms of iron homeostasis in erythroid precursors and cardiomyocytes. Hepcidin blocks the absorption of iron from the duodenum and the release of iron from macrophages and hepatocytes. Iron is bound to transferrin as ferric ion (Fe3+) and is internalized when transferrin docks with the transferrin receptor (transferrin receptor protein 1). Iron is released into the cytosolic pool in its reactive form (Fe2+), where it is available to be utilized by mitochondria in the synthesis of heme and iron–sulfur clusters. Cytosolic levels of Fe2+ are maintained in a tight range by the coordinated actions of iron regulatory proteins 1 and 2 (IRP1 and IRP2) and ferritin. Iron regulatory proteins stimulate TfR1 if cytosolic Fe2+ is low. Conversely, if Fe2+ increases, Fe2+ is sequestered as Fe3+ in a ferritin cage, which releases iron back into the cytosol by nuclear receptor coactivator 4 (NCOA4)‐mediated ferritinophagy. If cytosolic level of highly reactive Fe2+ increases excessively, the resulting production of reactive oxygen species oxidizes membrane‐bound phospholipids to promote ferroptosis; this oxidation and the resulting ferroptosis is prevented by glutathione peroxidase 4 (GPX4).
