FIGURE 1.
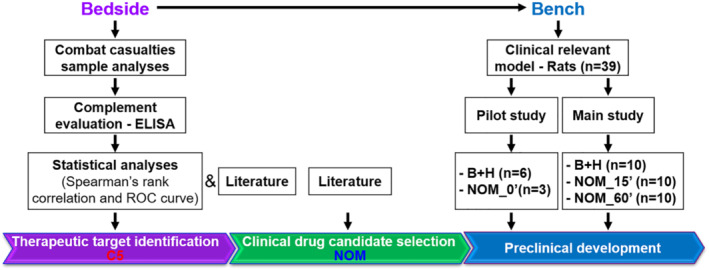
Workflow of translational study design. Blood plasma from 54 casualties on admission, 8 and 24 h after admission to a hospital and 10 civilian volunteers was used for the analysis of the complement activation. On the basis of the products of complement activation in the casualties' plasma, complement component C5 was identified as a reasonable therapeutic target. Prophylactic and therapeutic effects of nomacopan, an inhibitor of C5, were tested in rat models of blast (B) and haemorrhage (H) injury. Abbreviation: NOM, nomacopan
