Figure 2.
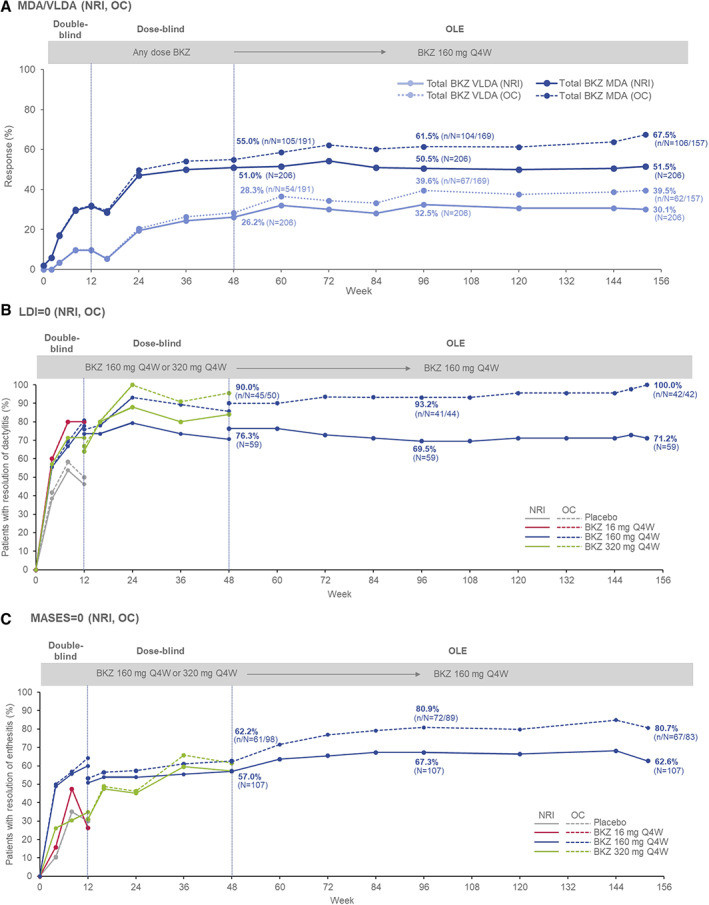
Additional efficacy outcomes in psoriatic arthritis patients from baseline (week 0) of the double‐blind period of the BE ACTIVE randomized controlled trial to week 152 (full analysis set). NRI and OC data are shown for the percentages of patients achieving minimal disease activity (MDA) or very low disease activity (VLDA) (A), percentages of patients achieving resolution of dactylitis based on Leeds Dactylitis Index (LDI) score (includes patients with LDI score >0 at baseline [n = 59]) (B), and percentages of patients achieving resolution of enthesitis based on the Maastricht Ankylosing Spondylitis Enthesitis Index (MASES) (includes patients with MASES score >0 at baseline [n = 107]) (C). Patients were classified as having MDA or VLDA when they met 5 of 7 or 7 of 7, respectively, of the following criteria: tender joint count score ≤1, swollen joint count score ≤1, PASI ≤1 or ≤3% body surface area affected by psoriasis, visual analog scale (VAS) score ≤15 for pain, VAS score ≤20 for patient global activity, Health Assessment Questionnaire disability index score ≤0.5, and tender entheseal points score ≤1. See Figure 1 for other definitions. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42280/abstract.
