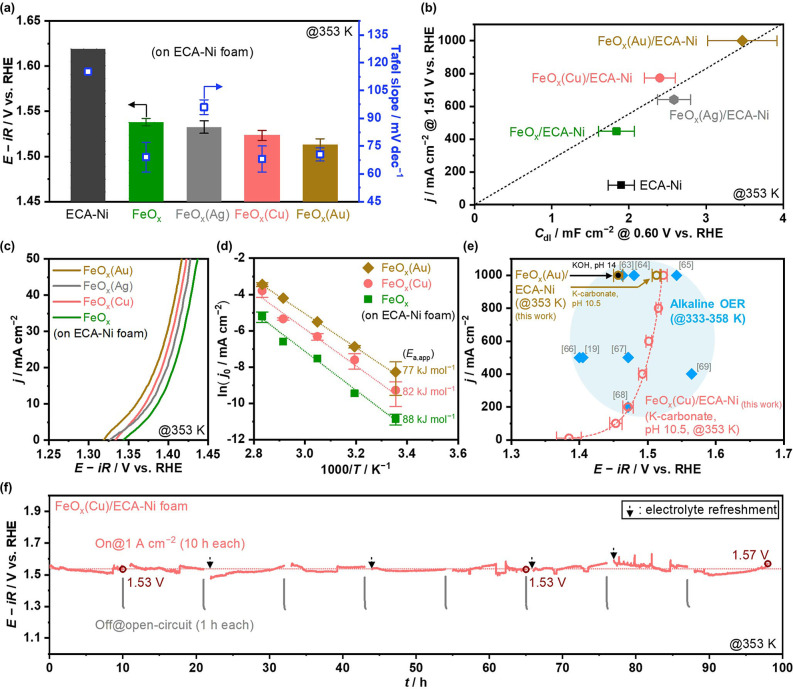
Figure 3.
OER performance over Fe‐based electrocatalysts on ECA−Ni foam. (a) Electrode potentials reaching +1 A cm−2 and Tafel slope in the range of current density of 10–1000 mA cm−2. (b) Relationship between C dl and current densities at 1.51 V vs. RHE. C dl were obtained at 0.60 V vs. RHE on CV (Figures S5, S7, and S16). Current densities were calculated from Tafel analysis (Figure S15). Dashed line represents a fixed specific current density. (c) LSV profiles at −1 mV s−1. (d) Arrhenius plot of the OER over ECA−Ni electrodes. The j 0 values were obtained from Tafel plot (Figure S18). (e) Current‐potential relationship over FeO x (Au)/ECA−Ni and FeO x (Cu)/ECA−Ni compared with reported performances in extremely alkaline conditions at 333–358 K,[ 19 , 63 , 64 , 65 , 66 , 67 , 68 , 69 ] where dashed line is current–potential curves calculated from Tafel analysis (Figure S15). (f) Potential profile during stability test over FeO x (Cu)/ECA−Ni, obtained by periodic CP at 1 A cm−2 for 10 h (On) with an interval of 1 h open‐circuit condition (Off). The solution was replaced with a fresh one sometimes because of the decrease in the amount of aqueous solution due to evaporation or/and consumption of water. All the electrochemical tests were conducted in 1.5 mol kg−1 K‐carbonate solution at pH 10.5 and 353 K, except for data of FeO x (Au) electrode in (e) that was obtained in KOH solution at pH 14.