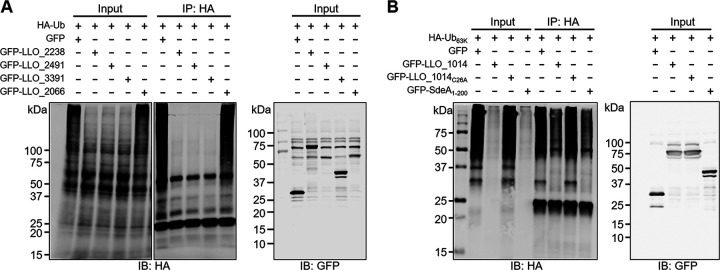
FIG 6.
L. longbeachae DUBs interfere with protein ubiquitination in mammalian cells. (A) HEK293T cells were cotransfected with a plasmid producing HA-Ub and plasmids encoding GFP-LLO_2238, GFP-LLO_2491, GFP-LLO_33911009–1200, or GFP-LLO_2066. Protein ubiquitination by HA-Ub was enriched by immunoprecipitation with anti-HA agarose and detected by Western blotting with the HA-specific antibody. The cell lysates were probed with a GFP-specific antibody to detect the expression of GFP fusion proteins. (B) HEK293T cells were transfected to coexpress HA-Ub63K and GFP-LLO_1014 or GFP-LLO_1014C26A. Cellular proteins modified by K63-linked polyubiquitination were immunoprecipitated with the anti-HA agarose and evaluated by Western blotting with the anti-HA antibody. Expression of GFP-LLO_1014 and GFP-LLO_1014C26A was detected by an anti-GFP antibody. GFP-SdeADUB was included as a positive DUB control. Data shown in panels A and B are one representative from three independent experiments.