FIG 2.
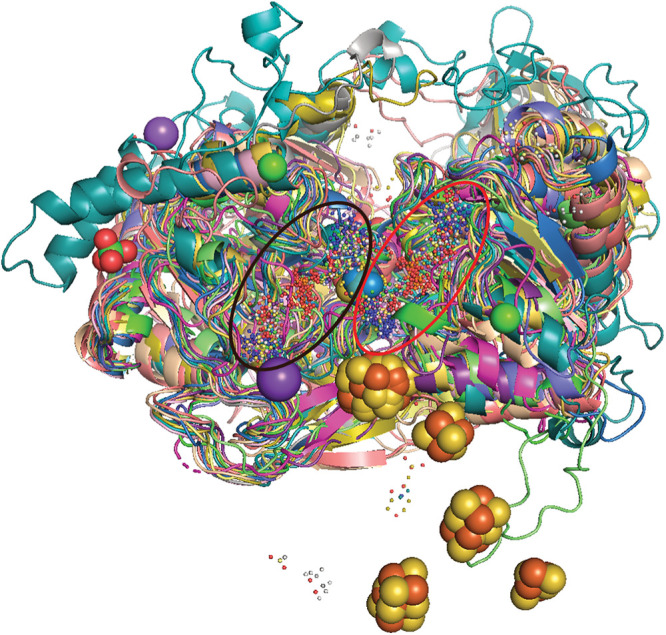
Superposition of all available crystal structures (of which there are 15) of MopB superfamily members and a single cryo-EM structure. The only portion that is retained is the region where the structural alignment of these structures was found to have significant structural homology. This region corresponds to the MopB domain; a stretch between the N-terminal iron-sulfur cluster, if present; and the PGD moiety of Mo/W-bisPGD proximal to that cluster. The proximal PGD is indicated by a black circle, and the distal PGD (the one furthest away) is indicated by a red circle. The cyan atoms at the intersection of these two circles represent Mo atoms, while the gold atoms represent W atoms. The various iron-sulfur clusters were retained in this image to demonstrate that while the orientation and positioning of the Mo/W-bisPGD cofactor of these diverse enzymes and catalytic subunits are conserved, the positionings of the iron-sulfur clusters differ substantially between different superfamily representatives.
