Keywords: chronic kidney disease, end-stage kidney disease, gout, hemodialysis, North America, peritoneal dialysis, prevalence
Abstract
Key Points
Gout is a common inflammatory arthropathy, and it can be as frequent in patients with ESKD as in the general adult population.
Patient outcomes and providers' practice patterns for ESKD patients with gout are unknown. We sought to address these gaps in this study.
We found that gout is not associated with worse clinical or patient-reported outcomes, despite being frequent and possibly under-reported.
Introduction
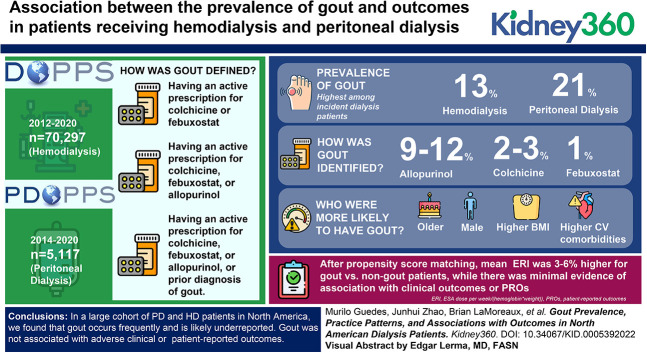
Gout occurs frequently in patients with kidney disease and can lead to a significant burden on quality of life. Gout prevalence, and its association with outcomes in hemodialysis (HD) and peritoneal dialysis (PD) populations located in North America, is unknown.
Methods
We used data from North America cohorts of 70,297 HD patients (DOPPS, 2012–2020) and 5117 PD patients (PDOPPS, 2014–2020). We used three definitions of gout for this analysis: (1) having an active prescription for colchicine or febuxostat; (2) having an active prescription for colchicine, febuxostat, or allopurinol; or (3) having an active prescription for colchicine, febuxostat, or allopurinol, or prior diagnosis of gout. Propensity score matching was used to compare outcomes among patients with versus without gout. Outcomes included erythropoietin resistance index (ERI=erythropoiesis stimulating agent dose per week/(hemoglobin×weight)), all-cause mortality, hospitalization, and patient-reported outcomes (PROs).
Results
The gout prevalence was 13% in HD and 21% in PD; it was highest among incident dialysis patients. Description of previous history of gout was rare, and identification of gout defined by colchicine (2%–3%) or febuxostat (1%) prescription was less frequent than by allopurinol (9%–12%). Both HD and PD patients with gout (versus no gout) were older, were more likely male, had higher body mass index, and had higher prevalence of cardiovascular comorbidities. About half of patients with a gout history were prescribed urate-lowering therapy. After propensity score matching, mean ERI was 3%–6% higher for gout versus non-gout patients while there was minimal evidence of association with clinical outcomes or PROs.
Conclusion
In a large cohort of PD and HD patients in North America, we found that gout occurs frequently and is likely under-reported. Gout was not associated with adverse clinical or PROs.
Introduction
Gout is an inflammatory response to monosodium urate crystals in the articular tissue, a frequently chronic and progressive condition driven by serum uric acid (SUA) above its solubility threshold of 6.8 mg/dl. Gout, the most common inflammatory arthropathy in adults, leads to increased health care utilization and poor health-related quality of life in the general population.1 Since the kidney excretes uric acid, SUA concentration increases as kidney function declines. Thus, the prevalence of both gout and hyperuricemia increases as GFR decreases.2 Gout inflicts patients with CKD five times more frequently than in age-standardized individuals with normal kidney function.2
Hyperuricemia correlates with increased endothelial cell oxidative stress, greater inflammation, and arterial stiffness and may contribute to accelerated atherosclerosis and poor cardiovascular (CV) outcomes in the general population.3,4 In observational studies among nondialysis CKD patients, both hyperuricemia and gout are associated with worse kidney outcomes5 and increased risk of CV mortality.6,7 In general, xanthine oxidase urate lowering therapy (ULT) agents safely and effectively treat gout in patients with CKD, despite the absence of available data from randomized controlled trials (RCTs) in patients with advanced CKD undergoing KRT.
A paucity of data exists on the prevalence of gout and its associations with clinical outcomes among patients with kidney failure (KF) undergoing KRT. Hemodialysis (HD) easily clears uric acid, a small nonprotein bound molecule. Peritoneal dialysis (PD), particularly continuous cycling PD, also effectively reduces SUA concentrations.8 Despite the rationale that KRT lowers SUA concentration through dialysis clearance and therefore reduces clinical significance of gout among patients with KF on dialysis, an observational study suggested an increased risk of all-cause mortality among ESKD patients with incident gout episodes.9 Notably, the cumulative incidence of gout in the dialysis population can be as high as the general population, suggesting that KRT may not reduce gout risk among patients with advanced CKD.9
Given the clinical importance of gout in patients with KF on dialysis, there remains an important unmet need to assess its prevalence and impact on key clinical and patient-reported outcomes (PRO), as well as to describe ULT practice patterns in KF populations. We sought to address these objectives to further characterize the clinical impact of gout on individuals living with KF undergoing KRT.
Materials and Methods
Study Design
This multicenter prospective cohort study used data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) and Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). The DOPPS is a continuous prospective cohort study of adult chronic in-center HD patients in 21 countries since 1996. Maintenance HD patients were randomly selected from HD facilities in each country. The PDOPPS is a continuous prospective cohort study designed to identify optimal practices for individuals treated with maintenance PD in eight countries since 2014. Adult patients were selected randomly for enrollment from stratified national samples of PD facilities.
Patient Population
This analysis uses US DOPPS data from phases 5–7 (2012–2020) and US PDOPPS data from phases 1–2 (2014–2020). The US DOPPS was approved by an independent institutional review board (E&I Study Number 98004-2, latest). The study was conducted according to the Declaration of Helsinki. Patient consent was obtained as required following national and local ethics committee regulations. Patients who did not have data on gout at enrollment were excluded. Details regarding DOPPS10 and PDOPPS11 cohorts have been previously published.
Exposure Variable
We used three definitions of gout for this analysis: (1) having an active prescription for colchicine or febuxostat; (2) having an active prescription for colchicine, febuxostat, or allopurinol; or (3) having an active prescription for colchicine, febuxostat, or allopurinol, or prior diagnosis of gout. Data on gout definitions were captured at the date of patient enrollment in PDOPPS or DOPPS.
History of gout was captured differently between PDOPPS and DOPPS. In DOPPS facilities and in large dialysis organization (LDO) PDOPPS clinics, comorbidity was defined by mapping international classification of disease (ICD)-10 codes entered by dialysis providers. In PDOPPS non-LDO facilities, medical history was assessed via a questionnaire completed by a study coordinator, with information on history of gout answered directly by the study coordinator on the basis of review of patient medical records.
Data were analyzed separately for each definition to explore sensitivity of gout prevalence and association with outcomes. Each exposure was represented as a dichotomous variable (reference group: patients without any of these indications of gout). We also used a five-category variable with mutually exclusive groups for (1) colchicine alone; (2) febuxostat; (3) allopurinol; (4) prior diagnosis of gout; and (5) none of the above.
Outcomes
Clinical Outcomes
We explored all-cause mortality, all-cause hospitalization, and CV death, as defined by the composite of CV-related codes for cause of death including acute myocardial infarction, cardiac arrhythmia, and stroke. In addition, we assessed baseline erythropoietin resistance index (ERI), defined as the erythropoiesis stimulating agent (ESA) weekly dose divided by (patient weight×hemoglobin). ERI is a surrogate for inflammatory activity in patients with CKD and has been associated with worse outcomes among patients with ESKD.12
Patient-Reported Outcomes
For health-related quality of life, the KDQOL-36 physical component summary (PCS) and mental component summary (MCS) were measured. We used the KDQOL-36, a disease-specific validated instrument in patients with ESKD, to assess symptoms and health-related quality of life among patients undergoing maintenance dialysis.13,14 The KDQOL-36 kidney-specific domains of burden, effects, and symptoms subscales were also analyzed. On the basis of prior studies of minimally important changes in KDQOL scores, a three- to five-point mean difference in PCS and MCS was considered clinically meaningful.15
Depression was assessed using the 10-item questionnaire Center for Epidemiologic Studies Depression Scale (CES-D) score, a validated instrument for depression screening in the general population.16,17 Functionality was measured by the Katz/Brody scale, which combines two validated measurements of basic and instrumental daily activities. In brief, the Katz/Brody scale assesses the respondent's ability to perform routine basic activities such as bathing, feeding themselves, and toileting, as well as instrumental activities, for example, financial, housekeeping, and shopping tasks. These measurements are strongly associated with health care utilization and are predictors of a poor prognosis in patients with ESKD.18,19 Finally, self-reported physical activity was defined by Rapid Assessment Physical Activity (RAPA), also a validated instrument in the general population.20 In summary, the RAPA allows physical activity categorization into low, moderate, and intense. Low physical activity, as defined by the RAPA, is associated with worse clinical outcomes in patients with CKD.
Among the HD cohort, limited data on PROs and cause of death were collected due to electronic health record (EHR) capture of US HD data, and thus, outcomes for HD were limited to all-cause mortality, hospitalization, and ERI.
Statistical Methods
Gout prevalence was estimated using the three gout definitions as above. For the associations with clinical and PROs, the most inclusive gout definition was used as the primary analysis. Associations between different gout definitions and outcomes were assessed via sensitivity analyses (Supplemental Figure 1).
We used propensity score matching to balance covariates between patients with and without gout. We matched patients with gout to non-gout patients using Proc PSMatch in SAS 9.4 using a 1:1 greedy nearest neighbor match with exact matching on sex, and a caliper of 0.5 for other variables that were used in the full model (listed below). Propensity score matching was done separately for HD and PD and for different outcomes.
Gout and Clinical Outcomes
We estimated the hazard ratio (HR) and 95% confidence interval (CI) of the association of gout with three clinical outcomes—(1) time to all-cause mortality; (2) time to first all-cause hospitalization; and (3) time to CV death—using Cox regression with a robust sandwich estimator to account for facility clustering. Follow-up began at study enrollment (when the exposure was assessed) and continued through the event of interest, namely, death, 7 days after leaving the facility due to transfer or change in modality, loss to follow-up, or administrative censoring at study end. Cox models were stratified by study phase and EHR data source. Propensity score models were fitted with progressive adjustments to demonstrate the impact of adjustment for potentially confounding covariates. Model 1 was unadjusted; model 2 included age; model 3 included sex, Black race, body mass index (BMI), and dialysis vintage; model 4 included comorbidities; model 5 included albumin, creatinine, and phosphorus; and finally, model 6 (HD only) included vascular access type.
Gout and Erythropoietin Resistance Index
To investigate whether patients with gout had greater ESA resistance, we estimated the difference in log (ERI) using linear generalized estimating equations (GEE) and reported the ratio of means (with 95% CI) separately for the HD and PD cohorts. Models have a random facility intercept to account for facility clustering. Progressive covariate adjustment following the models defined above was performed.
Gout and PROs
For continuous measures of health-related quality of life (i.e., PCS, MCS, kidney disease burden, kidney effects, and kidney disease symptoms), we estimated the difference (95% CI) in score using linear GEE, with repeated facility statement to account for facility clustering. For binary outcomes (i.e., CESD-10 score ≥10 [indicative of depressive symptoms], functional status score of <11 [indicative of some functional dependence], and inactive [versus highly active] RAPA score), we estimated the odds ratio (OR; 95% CI) using logistic regression, with GEE used to account for facility clustering.
Treatment of Missing Data
For missing data, we used the Sequential Regression Multiple Imputation Method implemented by IVEware and analyzed using the MIAnalyze procedure in SAS/STAT 9.4. All analyses used SAS software, version 9.4 (SAS institute, Cary, NC).
Results
Patient Characteristics
There were 70,297 HD patients and 5117 PD patients available in North America for analysis. Figure 1 depicts the inclusion and exclusion processes of this study. Patient characteristics before propensity score matching stratified by gout and by KRT modality are shown in Table 1.
Figure 1.
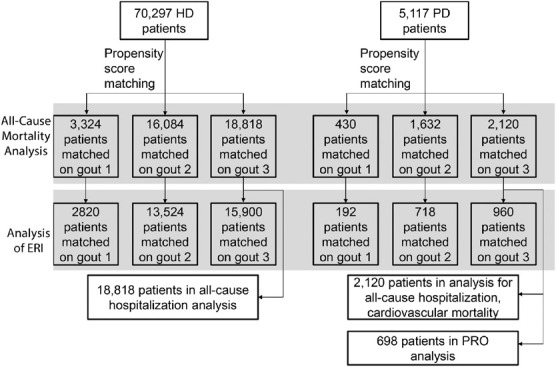
Study flowchart.
Table 1.
Patient characteristics in hemodialysis and peritoneal dialysis patients, with and without gout, before propensity score matching
| Characteristics | HD | PD | ||
|---|---|---|---|---|
| No Gout | Gout | No Gout | Gout | |
| Number of patients | 60,888 | 9409 | 4057 | 1060 |
| Demographics | ||||
| Patient age, yr | 63.2 (14.9) | 69.1 (12.8) | 56.2 (15.3) | 62.6 (13.7) |
| Male | 55% | 64% | 53% | 69% |
| Black race | 39% | 35% | 27% | 25% |
| BMI, kg/m2 | 28.3 (7.1) | 30.0 (7.3) | 29.0 (6.5) | 30.3 (6.2) |
| Time on dialysis, years | 3.35 (4.04) | 2.27 (2.97) | 2.63 (3.39) | 2.20 (2.83) |
| Comorbidity history | ||||
| Coronary artery disease | 19% | 26% | 18% | 25% |
| Cancer (nonskin) | 5% | 9% | 5% | 10% |
| Other cardiovascular disease | 16% | 26% | 10% | 15% |
| Cerebrovascular disease | 6% | 7% | 4% | 6% |
| Congestive heart failure | 24% | 32% | 12% | 16% |
| Diabetes | 66% | 64% | 52% | 48% |
| Gastrointestinal bleeding | 4% | 4% | 1% | 3% |
| Hypertension | 79% | 83% | 75% | 79% |
| Lung disease | 7% | 11% | 4% | 6% |
| Neurologic disease | 5% | 6% | 2% | 3% |
| Psychiatric disorder | 24% | 25% | 19% | 19% |
| Peripheral vascular disease | 12% | 14% | 9% | 10% |
| Recurrent cellulitis/gangrene | 5% | 5% | 2% | 2% |
| Dialysis treatments | ||||
| Vascular access type | ||||
| Catheter | 41% | 37% | ||
| Fistula | 47% | 51% | ||
| Graft | 13% | 12% | ||
| Single-pool Kt/V | 1.54 (0.28) | 1.53 (0.28) | ||
| Laboratory results | ||||
| Phosphorus, mg/dl | 5.19 (1.64) | 5.06 (1.49) | 5.31 (1.56) | 5.08 (1.44) |
| Albumin, g/dl | 3.63 (0.49) | 3.65 (0.46) | 3.51 (0.47) | 3.54 (0.43) |
| PTH, pg/ml | 322 [188–535] | 312 [187–500] | 358 [210–556] | 346 [206–520] |
| Creatinine, mg/dl | 7.57 (3.07) | 7.11 (2.90) | 9.04 (4.30) | 8.62 (4.14) |
| Hemoglobin, g/dl | 10.5 (1.4) | 10.6 (1.4) | 11.0 (1.5) | 11.1 (1.5) |
| Ferritin, ng/ml | 615 [338–948] | 603 [328–941] | 582 [300–933] | 549 [271–939] |
| White blood cell count, 103 cells/mm3 | 6.7 [5.4–8.4] | 6.8 [5.4–8.5] | 7.2 [5.7–9.2] | 7.3 [5.7–9.2] |
Gout: active Rx for colchicine or febuxostat or allopurinol; or prior diagnosis of gout (Gout3); mean (SD), median [interquartile range], or % shown. Most variables had missingness <15%, with exception of Black (18%), VA type (16%), and single-pool Kt/V (27%) in HD, and BMI (28%), PTH (17%), and white blood cell count (23%) in PD patients. HD, hemodialysis; PD, peritoneal dialysis; PTH, parathyroid hormone; BMI, body mass index.
The proportion of patients missing information on prior gout diagnosis was 14% in HD and 1% in PD. The proportion of patients with missing medication information was 5% in HD and 14% in PD. Missingness for most covariates was low (<20%), except for BMI (28%) in PD.
PD patients were younger than HD patients and with a generally lower comorbidity burden. Both HD and PD patients with gout were older, were more likely male, had a higher BMI, had a shorter dialysis vintage, and had a higher prevalence of comorbidities than patients without gout. Levels of serum albumin, serum phosphorus, and hemoglobin varied minimally by gout status. In HD, patients with gout were more likely to dialyze with a fistula than catheter. Patient characteristics stratified by gout medication use are described in Supplemental Tables 1a and 1b. After propensity score matching, all measured covariates were well balanced (Supplemental Table 2).
Gout Prevalence
Gout prevalence varied depending on the exact definition. Considering patients with a current prescription for colchicine or febuxostat, gout prevalence was 2% in HD and 4% in PD. Adding allopurinol prescription to the definition increased the estimates to 11% in HD and 16% in PD. Finally, including history of gout to the definitions above increased gout prevalence to 13% in HD and 21% in PD (Figure 2). Gout prevalence decreased with longer dialysis vintage for both HD and PD patients (Figure 2), whereas it was higher when gout medical history was assessed by medical questionnaire than through ICD-10 mapping (Figure 2). Among HD and PD patients with gout in their medical history, 42% and 48% were prescribed at least one ULT, respectively.
Figure 2.
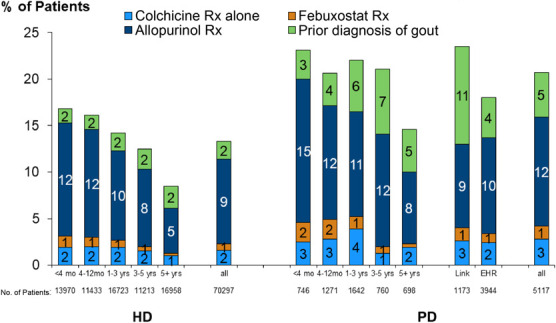
Gout prevalence in US HD and PD patients. DOPPSLink: prior diagnosis of gout on the basis of yes/no question completed by study coordinator; EHR: prior diagnosis of gout on the basis of ICD-10 codes. Mutually exclusive categories were defined hierarchically as (1) colchicine Rx alone; (2) febuxostat Rx with no colchicine Rx; (3) allopurinol Rx with no colchicine or febuxostat Rx; (4) prior diagnosis of gout with no colchicine, febuxostat, or allopurinol Rx; and (5) none of the above.
Clinical Outcomes
Among all 70,297 HD patients, 11,739 died (17%) during follow-up (median: 11 months, interquartile range [IQR], 4–23 months). After implementing the propensity score matching, we matched all 9409 HD patients with gout 1:1 to patients with no indication of gout. In this subset of 18,818 patients eligible for analyses of outcomes, 3492 died (19%) during a median follow-up of 11 months. Among all 5117 PD patients, 546 died (11%) during follow-up (median, 9 months; IQR, 4–19 months). After implementing the propensity score matching, we matched all 1060 PD patients with gout 1:1 to patients with no indication of gout. In this subset of 2160 patients eligible for analyses of outcomes, 269 died (13%) during a median follow-up of 9 months.
Figure 3, A and B, illustrates the propensity score-matching analysis by gout definitions on all-cause mortality. In the fully adjusted models, the HR (95% CI) of all-cause mortality for gout (versus non-gout) was 0.94 (0.87 to 1.02) in HD and 0.95 (0.71 to 1.28) in PD (Figure 3).
Figure 3.
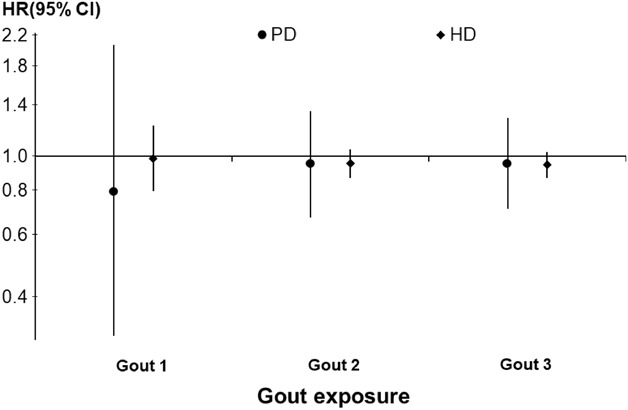
Association between gout and all-cause mortality among HD and PD patients. Binary exposure definitions: (1) Gout1: active Rx for colchicine or feboxustat; (2) Gout2: active Rx for colchicine or feboxustat or allopurinol; and (3) Gout3: active Rx for colchicine or feboxustat or allopurinol; or prior diagnosis of gout. Cox models were stratified by study phase and EHR data source, and propensity-score matched 1:1 with progressive covariate adjustments model 1: unadjusted; model 2: age only; model 3: model 2 + other demographics, including sex, Black race, BMI, and dialysis vintage; model 4: model 3 + 13 comorbidities; model 5: model 4 adjustments+albumin, creatinine, and phosphorus; model 6 (HD only): model 5 adjustments+vascular access type. Estimates from the fully adjusted models are reported. BMI, body mass index.
The adjusted HR (95% CI) of CV-related death for gout versus non-gout was 1.08 (0.60 to 1.96) in PD (no cause of death data for HD). The adjusted HR (95% CI) of all-cause hospitalization for gout versus non-gout was 1.01 (0.96 to 1.06) in HD and 1.06 (0.91 to 1.23) in PD. Results according to distinct gout definitions did not qualitatively change the estimates (Figure 3). Supplemental Figure 1 depicts additional sensitivity analyses by gout definition.
Erythropoietin Resistance Index
The median (IQR) ERI was 13.2 unit/wk per g/dl per kg (7.2–23.1) in HD patients and 8.5 (4.5–15.5) in PD patients. Crude analyses of the HD cohort showed that the median (IQR) ERI was 12.7 (6.8–21.6) among gout patients versus 13.3 (7.3–23.3) among non-gout patients. In PD, the median (IQR) ERI was 8.2 (4.5–14.5) among gout patients versus 8.6 (4.5, 15.8) among non-gout patients. In propensity score–matched models, the ratio of ERI means (95% CI) between gout and non-gout patients was 1.06 (1.03 to 1.09) in HD and 1.03 (0.90 to 1.18) in PD, that is, mean ERI was 6% higher in the HD cohort and 3% higher in the PD cohort in gout versus non-gout patients. Figure 4 depicts stepwise model estimates for the association between gout and ERI.
Figure 4.
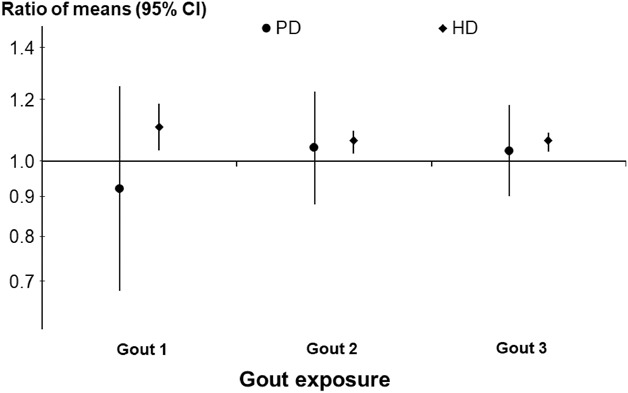
Associations between gout and ERI among HD and PD patients. Estimates from the fully adjusted models are reported.
Patient-Reported Outcomes
Continuous PRO measures are summarized in Table 2. For the physical and mental component summary scores, adjusted models showed that PD patients with gout had 0.7 point lower mean PCS and 0.6 point lower MCS, although estimates were generally imprecise. Null results were similarly observed for other measures of PRO including the burden, effects, and symptoms of kidney disease (Table 2).
Table 2.
Association between gout and HRQOL in peritoneal dialysis
| Outcome | Overall Mean | Among Gout | Among No-Gout | Effect | 95% CI |
|---|---|---|---|---|---|
| PCS | 37.5 | 37.4 | 37.5 | −0.7 | −2.3 to 0.9 |
| MCS | 49.0 | 48.7 | 49.3 | −0.6 | −2.2 to 1.1 |
| Kidney disease burden | 54.7 | 55.2 | 54.3 | −0.8 | −5.8 to 4.2 |
| Kidney disease effects | 72.8 | 73.4 | 72.3 | 1.0 | −2.2 to 4.2 |
| Kidney disease symptoms | 78.0 | 77.9 | 78.1 | −0.4 | −2.6 to 1.8 |
Adjusted difference between gout and non-gout patients (Gout3). Propensity score models adjusted for study phase, EHR data source, demographics, including patient age, sex, Black race, BMI, and dialysis vintage, 13 comorbidities, albumin, creatinine, phosphorus, and vascular access type. HRQOL, health-related quality of life; CI, confidence interval; PCS, physical component summary; MCS, mental component summary EHR, electronic health record; BMI, body mass index.
Other binary PROs are summarized in Table 3. The adjusted OR (95% CI) comparing gout versus non-gout PD patients was 0.85 (0.57 to 1.27) for CES-D score ≥10 (indicative of depression), 0.94 (0.56 to 1.59) for functional status score <11 (indicative of some functional dependence), 0.91 (0.63 to 1.32) for RAPA-strength/flexibility, and 1.29 (0.78 to 2.14) for RAPA-aerobic (Table 3).
Table 3.
Association between gout and other patient-reported outcomes in peritoneal dialysis
| Outcome | Overall Prevalence, % | Among Gout, % | Among No-Gout, % | OR | 95% CI |
|---|---|---|---|---|---|
| CESD-10 score ≥10 | 22 | 21 | 22 | 0.85 | 0.57 to 1.27 |
| Functional status score of <11 | 20 | 19 | 20 | 0.94 | 0.56 to 1.59 |
| RAPA strength/flexibility score, none versus other | 65 | 61 | 65 | 0.91 | 0.63 to 1.32 |
| RAPA aerobic score, not active versus active | 18 | 19 | 18 | 1.29 | 0.78 to 2.14 |
OR between gout and non-gout patients (Gout3). Propensity score models adjusted for study phase, EHR data source, demographics, including patient age, sex, Black race, BMI, and dialysis vintage, 13 comorbidities, albumin, creatinine, and phosphorus. RAPA strength/flexibility score was dichotomized as none versus other (strength but not flexibility, flexibility but not strength, or both). RAPA aerobic score was dichotomized as not active (never/rarely active or infrequently active) versus active (sometimes active, often active, or very active). OR, odds ratio; CI, confidence interval; CESD-10, Center for Epidemiologic Studies Depression-10; RAPA, Rapid Assessment Physical Activity; EHR, electronic health record; BMI, body mass index.
Discussion
In this nationwide cohort study in North America, we found that gout is a common yet under-reported comorbidity among HD and PD patients. Less than half of the patients with gout are prescribed ULT agents. Allopurinol was the most common prescribed ULT agent in this population. Gout prevalence as indicated by medical history or drug therapy was not found to be associated with worse clinical or PROs.
In our study, gout prevalence, despite varying by specific definitions, was 13% for HD and 21% in PD. Gout prevalence was similarly high in a cohort of patients in New Zealand that found a prevalence of 28% among KF on KRT.21 These estimates are akin to studies in the nondialysis CKD population, wherein prevalence varied between 16% and 36% depending on GFR. We found that gout patients were on average older, had higher BMI, and had greater CV comorbidity burden. These results are consistent with previous reports both in nondialysis CKD22,23 and in dialysis patients.24 Also similar in HD and PD populations, longer dialysis vintage was associated with decreasing gout prevalence.21
Between KRT modalities and across dialysis vintage, allopurinol was the most prescribed ULT agent, as compared with febuxostat. Although there are few RCTs of xanthine oxidase inhibitors in patients with CKD, both allopurinol and febuxostat are considered safe in the CKD population.25 A recent RCT in patients with moderate to advanced CKD showed allopurinol safely and effectively reduces SUA levels, but it did not improve clinical outcomes.26 In our study, approximately half of HD and PD patients with a history of gout were receiving ULTs. Despite the belief that KRT effectively reduces SUA levels and gout flares, observational studies in the HD population estimated a 15% gout incidence9 over 5 years, a similar rate compared with the general population.27 Therefore, our results may point to the under prescription of ULT in ESKD patients with gout.28
Additional factors may play a role in the relatively low proportion of ULT prescription among patients with KF on dialysis with a gout medical history. Increased uric acid production in CKD results from ingesting high-purine foods or taking medications associated with hyperuricemia. Frequently used medications that cause hyperuricemia include angiotensin converting enzyme inhibitors (ACEi) and diuretics.29,30 As KF ensues, deprescription of these drugs may reduce SUA and reduce gout episodes. In fact, we found higher gout prevalence among PD patients, who are often prescribed diuretics for volume control and ACEi for preserving peritoneal membrane and residual kidney function.31 Finally, it remains possible that KF patients with gout medical history are actively tapered off ULTs over time. Currently, there are no clear recommendations to deprescribe ULT in KF patients with gout who remain free of flares, although individualization balancing patients' preferences are recommended for the general population.32
Our results do not support the hypothesis that gout portends worse clinical and PROs in patients with KF on dialysis. A previous gout medical history or its treatment was not associated with worse scores on depression, functionality, general health-related quality of life, or self-reported physical activity. In addition, risks of all-cause mortality and hospitalization were not different across our propensity score–matched cohorts. This finding contrasts with a United States Renal Data System (USRDS) analysis9 that reported incident gout associated with increased mortality and CV hospitalization risk in HD patients. Of note, the USRDS study did not adjust for important key confounders such as laboratory variables and vascular access. In addition, we analyzed associations between prevalent gout rather than incident episodes. Specifically, prevalent maintenance dialysis patients are often chronically inflamed and malnourished. This phenotype is associated with lower SUA, and selection bias of CKD patients onto chronic dialysis can affect the associations between SUA, gout, and mortality33—similar to the paradox of obesity associated with lower mortality in the dialysis population.
Both asymptomatic hyperuricemia and gout activate inflammatory pathways.34 As a surrogate outcome, we explored the associations between ERI, which associates with inflammatory states. Our results suggest gout increases ESA resistance in patients with KF, a potential indicator of residual inflammation driving worse anemia outcomes. However, the effect size of this association was small and in PD patients our wide confidence intervals restrict interpretability. The clinical significance of these results, albeit potentially limited, warrants further analyses.
Of note, our study has important limitations. Our prevalence estimates varied according to the definition of gout. In the most comprehensive gout definition, we used allopurinol, febuxostat, colchicine, and gout medical history as gout indicators. However, colchicine can be prescribed for reasons other than gout flares, despite being used by only up to 3% of our cohort. Albeit contraindicated in advanced CKD, colchicine can be prescribed for other crystal arthropathies, such as calcium pyrophosphate deposition (CPPD)–related disorders, which is also common in CKD.35 Allopurinol is also often prescribed for other reasons than gout in CKD. Of particular interest is asymptomatic hyperuricemia, a common and frequently treated comorbidity in CKD. Because of such heterogeneity in practice patterns, it is difficult to predict whether our gout estimates on the basis of ULT prescription are underestimated or overestimated.
Other key limitations are noteworthy. Because of the observational design, we cannot rule out residual confounding. Moreover, SUA levels were not routinely measured in this population and were only available in a small subset of patients (<10%) for whom they were measured by indication; thus, we were unable to describe how many patients with gout had SUA levels within clinically recommended targets. We also did not have data on acute gouty arthritis episodes or gout severity, which can limit the interpretation of our findings.
Finally, distinct data extraction mechanisms in our cohorts may have affected our estimates. In facilities where a medical questionnaire was used to define comorbidities, gout was more frequently reported. In our study, medical questionnaires were used by non-LDO and small dialysis facilities, whereas EHR-based ICD-10 mapping was the primary method used to define gout in LDO facilities. We have no reason to believe that gout patients distribute unevenly across LDO versus non-LDOs or small dialysis facilities; therefore, these findings are probably due to under-reporting in EHR data extraction. This issue was previously reported and reflects the challenges in analyzing observational data from EHR sources.36 To the authors' knowledge, the use of ICD-10 codes has not yet been validated among patients with ESKD. Patients on dialysis are often affected by conditions that can closely mimic gout, such as calcific arthritis, CPPD, and osteoarthritis, all frequent in the context of hyperuricemia. This resulting diagnostic uncertainty can affect the accuracy of prevalence estimates in this population, irrespective of the data capture mechanism. In sum, the combination of gout under-reporting, potential misdiagnosis, and the use of active gout treatment to ascertain gout exposure may have shifted our findings toward the null. These results may help inform future study designs leveraging real-world data in populations with gout.
This study also has relevant strengths. This is the first analysis in a representative nationwide cohort of both PD and HD patients to address gout prevalence, practice patterns, and clinical associations in North America. Particularly, we reported a comprehensive set of PROs, key to the outstanding need of exploring factors affecting health-related quality of life to meet the care expectations of patients with KF. Our estimates were robust across many sensitivity analyses, including different exposure definitions and the inclusion of key confounders after propensity score matching.
Our null findings for PROs do not rule out short-term impacts on health related quality of life driven by preventable acute gouty arthritis episodes. Gout is recurrent and progressive, often leading to debilitating pain in a subset of highly burdened patients. The natural history of gout in KF remains elusive as observational studies have shown a high incidence of acute gouty arthritis among patients with ESKD without a prior diagnosis of gout.9 Efforts should thus be furthered to characterize gout natural history in ESKD, as well as to increase awareness of de novo cases after dialysis initiation to prevent short-term morbidity and avoid unnecessary interventions that may expose patients with KF to adverse events.
Finally, in this large cohort of PD and HD patients in North America, we found that gout is a frequent, likely under-reported comorbidity. Yet, a history of gout or its pharmacological treatment is not associated with worse clinical outcomes or PROs. Given its recurrent and progressive nature, there remains a significant gap in the understanding of gout clinical course to guide medical practice in patients with KF undergoing KRT.
Supplementary Material
Acknowledgments
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance for this paper.
Footnotes
*DOPPS 7 (2018–2021) Country Investigators: The DOPPS is led by the following investigators in each participating country: Ali Alaradi (Bahrain); Pieter Evenepoel, Michel Jadoul (Belgium); Manish Sood, Rita Suri (Canada); Xiaonong Chen, Yuqing Chen, Fanfan Hou, Xinling Liang, Zhaohui Ni, Li Zuo (China); Christian Combe, Fitsum Guebre-Egziabher, Pablo Antonio Ureña Torres (France); Werner Kleophas, Elke Schaeffner, Thomas Weinreich (Germany); Giuliano Brunori, Loreto Gesualdo, Francesco Locatelli, (Italy); Masafumi Fukagawa, Masaaki Inaba, Masaomi Nangaku, Kosaku Nitta, Kazuhiko Tsuruya (Japan); Saeed Al‐Ghamdi, Mohammed Al Ghonaim, Fayez Hejaili, Ayman Karkar, Faissal Shaheen, Jamal Al Wakeel (Kingdom of Saudi Arabia); Naser Alkandari, Anas Alyousef, Bassam Al Helal (Kuwait); Issa Alsalmi, Yacoub Al Maimani (Oman); Fadwa Al Ali, Abdulla Hamad (Qatar); Aleix Cases, Almudena Vega Martínez, Patricia de Sequera (Spain); Anders Christensson, Stefan Jacobson (Sweden); Samra Abouchacra, Mohamed Hassan, Ali Abdulkarim Al Obaidli, Mona Al Rukhaimi, Abdul Kareem Saleh (United Arab Emirates); Elham Asgari, Indranil Dasgupta, Hugh Rayner (United Kingdom). Country Investigators from prior DOPPS study phases can be found at: https://www.dopps.org/OurStudies/HemodialysisDOPPS.aspx. PDOPPS Steering Committee members: David Johnson (Australia); Jeffrey Perl (Canada); Mauricio Sanabria (Colombia);Hideki Kawanishi (Japan); Yong-Lim Kim (South Korea); Talerngsak Kanjanabuch (Thailand); Simon Davies (United Kingdom); Ronald Pisoni, Bruce Robinson, Jenny Shen (United States).
Disclosures
V. Domingues declares stocks ownership in Abbvie, Aurinia pharma, Bristol Mayers, Horizon, Lilly, Novartis, Pfizer and payment for speaker bureau: Abbvie, Aurinia, BMY, Exagen, GSK, Horizon, Lilly, and Novartis. B. Gorlitsky declares Speakers Bureau/Honoraria from Horizon. A. Karaboyas, R. Pecoits-Filho, B. Robinson, and J. Zhao are employees of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Program is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. B. LaMoreaux is an employee and holds stock in Horizon Therapeutics. B. Marder is an employee and holds stock in Horizon Therapeutics. R. Pecoits-Filho also declares Honorarium (paid to employer) from AstraZeneca, Boehringer-Lilly, Novo Nordisk, Akebia, Bayer for participation in advisory Boards and payment or reimbursement of travel/accommodation expenses for expert testimony or lectures (including service on speakers' bureaus): Honorarium (paid to employer) from AstraZeneca, Boehringer-Lilly, Novo Nordisk, Akebia, Bayer for participation in educational activities. M.B. Rivara declares grant funding from Satellite Healthcare and Honoraria from the International Society of Peritoneal Dialysis and the National Kidney Foundation. B. Robinson has received consultancy fees or travel reimbursement since 2019 from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin Co., all paid directly to his institution of employment. All remaining authors have nothing to disclose.
Funding
This work was directly supported by Horizon Therapeutics USA, Inc. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details, see https://www.dopps.org/AboutUs/Support.aspx.
Author Contributions
B. Robinson conceptualized the study; R. Pecoits-Filho and B. Robinson were responsible for methodology; V. Domingues, M. Guedes, B. LaMoreaux, S. Lew, B. Marder, R. Pecoits-Filho, M.B. Rivara, and B. Robinson were responsible for investigation; M. Guedes, A. Karaboyas, B. Marder, R. Pecoits-Filho, B. Robinson, and J. Zhao wrote the original draft; and M. Guedes, J. Zhao, B. LaMoreaux, B. Marder, B. Gorlitsky, V. Domingues, M.B. Rivara, S. Lew, B. Robinson, R. Pecoits-Filho, and A. Karaboyas reviewed and edited the manuscript.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A236.
Supplemental Table 1A. HD patient characteristics, by gout indications.
Supplemental Table 1B. PD patient characteristics, by gout indications.
Supplemental Table 2. Patient characteristics in HD and PD patients, with and without gout, after propensity score matching.
Supplemental Figure 1. Association between five-category gout exposure and all-cause mortality in HD (A) and PD (B).
References
- 1.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2007;67(9):1310–1316. doi: 10.1136/ard.2007.081604 [DOI] [PubMed] [Google Scholar]
- 2.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009-10. PLoS One. 2012;7(11):e50046. doi: 10.1371/journal.pone.0050046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Lozada LG Soto V Tapia E, et al. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–F1141. doi: 10.1152/ajprenal.00104.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17(6):409–414. doi: 10.1016/j.numecd.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 5.Oh TR Choi HS Kim CS, et al. Hyperuricemia has increased the risk of progression of chronic kidney disease: propensity score matching analysis from the KNOW-CKD study. Sci Rep. 2019;9(1):6681. doi: 10.1038/s41598-019-43241-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CL, Tsai SF. Association between mortality and serum uric acid levels in non-diabetes-related chronic kidney disease: an analysis of the National Health and Nutrition Examination Survey, USA, 1999-2010. Sci Rep. 2020;10(1):17585. doi: 10.1038/s41598-020-74747-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyaoka T, Mochizuki T, Takei T, Tsuchiya K, Nitta K. Serum uric acid levels and long-term outcomes in chronic kidney disease. Heart Vessels. 2014;29(4):504–512. doi: 10.1007/s00380-013-0396-0 [DOI] [PubMed] [Google Scholar]
- 8.Diez-Lopez C, Perez-Contreras J, Andres M. Urate levels and clearance in renal patients under peritoneal dialysis. Nucleosides Nucleotides Nucleic Acids. 2021;40(7):720–731. doi: 10.1080/15257770.2021.1934482 [DOI] [PubMed] [Google Scholar]
- 9.Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19(11):2204–2210. doi: 10.1681/ASN.2007111256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5 suppl 2):7–15. doi: 10.1053/j.ajkd.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 11.Perl J Davies SJ Lambie M, et al. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int. 2016;36(3):297–307. doi: 10.3747/pdi.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaboyas A Morgenstern H Fleischer NL, et al. Inflammation and erythropoiesis-stimulating agent response in hemodialysis patients: a self-matched longitudinal study of anemia management in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Med. 2020;2(3):286–296. doi: 10.1016/j.xkme.2020.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peipert JD, Bentler PM, Klicko K, Hays RD. Psychometric properties of the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36) in the United States. Am J Kidney Dis. 2018;71(4):461–468. doi: 10.1053/j.ajkd.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 14.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the Kidney Disease Quality of Life (KDQOLTM) Instrument. Qual Life Res. 1994;3(5):329–338. doi: 10.1007/bf00451725 [DOI] [PubMed] [Google Scholar]
- 15.Li JW Yin D Wang Z, et al. New-onset gout as an independent risk factor for returning to dialysis after kidney transplantation. Transplant Direct. 2020;6(12):e634. doi: 10.1097/TXD.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a Short Form of the CES-D. Am J Prev Med. 1994;10(2):77–84. doi: 10.1016/s0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 17.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3 pt 1):179–186. doi: 10.1093/geront/9.3_part_1.179 [DOI] [PubMed] [Google Scholar]
- 19.Jassal SV Karaboyas A Comment LA, et al. Functional dependence and mortality in the international Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2016;67(2):283–292. doi: 10.1053/j.ajkd.2015.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 21.Yeo E, Palmer SC, Chapman PT, Frampton C, Stamp LK. Serum urate levels and therapy in adults treated with long-term dialysis: a retrospective cross-sectional study. Intern Med J. 2019;49(7):838–842. doi: 10.1111/imj.14163 [DOI] [PubMed] [Google Scholar]
- 22.Jing J Kielstein JT Schultheiss UT, et al. ; GCKD Study Investigators. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant. 2015;30(4):613–621. doi: 10.1093/ndt/gfu352 [DOI] [PubMed] [Google Scholar]
- 23.Yokose C, Chen M, Berhanu A, Pillinger MH, Krasnokutsky S. Gout and osteoarthritis: associations, pathophysiology, and therapeutic implications. Curr Rheumatol Rep. 2016;18(10):65. doi: 10.1007/s11926-016-0613-9 [DOI] [PubMed] [Google Scholar]
- 24.Bleyer AJ, Zhang Y, Ksirsagar O, Marder B, LaMoreaux B. Risk factors and outcomes of gout in dialysis patients from the United States Renal Data System (USRDS). Am J Kidney Dis. 2022;79(4):S15. [Google Scholar]
- 25.Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis. 2017;70(3):422–439. doi: 10.1053/j.ajkd.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badve SV Pascoe EM Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382(26):2504–2513. doi: 10.1056/NEJMoa1915833 [DOI] [PubMed] [Google Scholar]
- 27.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–390. doi: 10.1038/s41584-020-0441-1 [DOI] [PubMed] [Google Scholar]
- 28.Doherty M Jansen TL Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71(11):1765–1770. doi: 10.1136/annrheumdis-2012-201687 [DOI] [PubMed] [Google Scholar]
- 29.Ben Salem C, Slim R, Fathallah N, Hmouda H. Drug-induced hyperuricaemia and gout. Rheumatology. 2017;56(5):679–688. doi: 10.1093/rheumatology/kew293 [DOI] [PubMed] [Google Scholar]
- 30.Choi HK, Soriano LC, Zhang Y, Rodriguez LAG. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012;344:d8190. doi: 10.1136/bmj.d8190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phatthanasobhon S Nochaiwong S Thavorn K, et al. Effectiveness of renin-angiotensin-aldosterone system blockade on residual kidney function and peritoneal membrane function in peritoneal dialysis patients: a network meta-analysis. Sci Rep. 2019;9(1):19582. doi: 10.1038/s41598-019-55561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzgerald JD Dalbeth N Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744–760. doi: 10.1002/acr.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latif W Karaboyas A Tong L, et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin J Am Soc Nephrol. 2011;6(10):2470–2477. doi: 10.2215/CJN.00670111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joosten LAB, Crişan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol. 2020;16(2):75–86. doi: 10.1038/s41584-019-0334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rho YH, Zhu Y, Zhang Y, Reginato AM, Choi HK. Risk factors for pseudogout in the general population. Rheumatology (Oxford). 2012;51(11):2070–2074. doi: 10.1093/rheumatology/kes204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Violán C Foguet-Boreu Q Hermosilla-Pérez E, et al. Comparison of the information provided by electronic health records data and a population health survey to estimate prevalence of selected health conditions and multimorbidity. BMC Public Health. 2013;13(1):251. doi: 10.1186/1471-2458-13-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.