Figure 1.
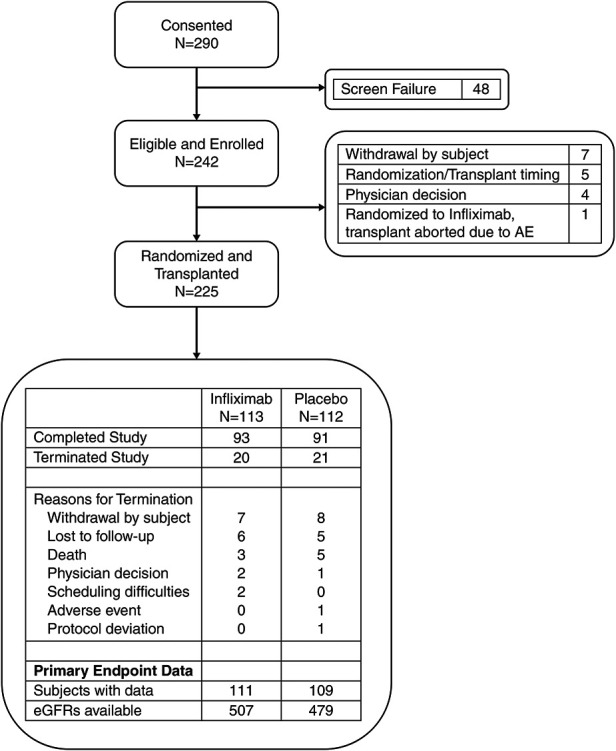
Two hundred twenty-five subjects were evaluable for the primary end point. Flow of all patients who provided consent through the study. Reasons for termination before and after transplant are provided. For randomized and transplanted patients, disposition is presented, along with a summary of data available for primary end point analysis. AE, adverse event.
