Fig. 1 |. Kap-mediated nucleocytoplasmic transport and principles of Kap–cargo interactions and RAN–GTP-dependent loading/unloading.
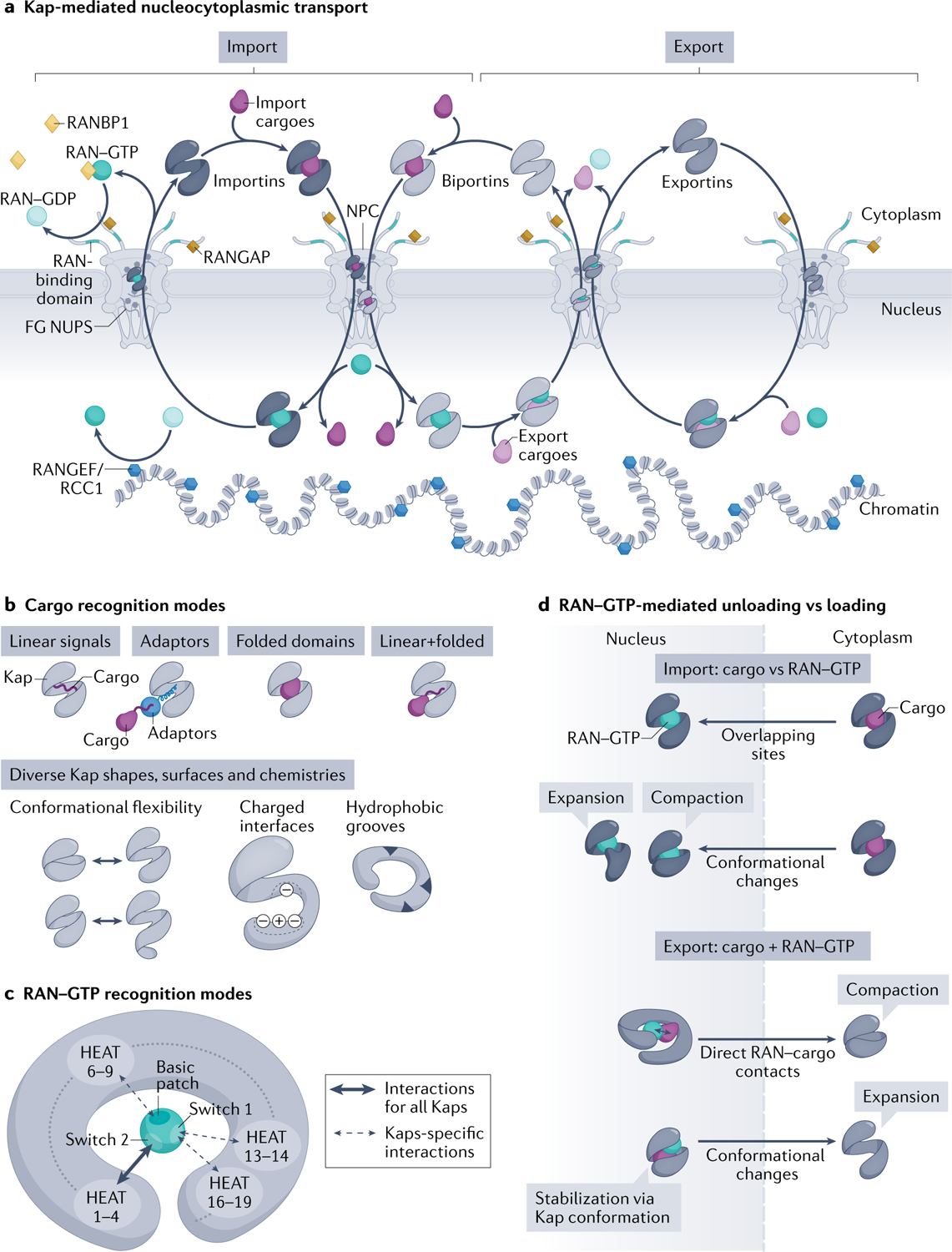
a | Karyopherins (Kaps) (comprising importins, biportins and exportins; shown in grey) and the RAN–GTP gradient control directional transport of cargoes across nuclear pore complexes (NPCs). RAN–GTP (dark teal circles) enriched in the nucleus by the action of chromatin-bound nucleotide exchange factor RANGEF/RCC1 (blue hexagons). Cytoplasmic RAN–GDP (light teal circles) enriched by action of cytoplasmic RAN GTPase-activating protein (RANGAP) (gold squares), RAN-binding domains in nucleoporin RANBP2 (teal fibrils) and cytosolic RANBP1 (yellow diamonds), which accelerate GTP hydrolysis. Importins (left) and biportins (middle) bind cytosolic import cargoes (dark purple), translocate across NPCs by interacting with phenylalanine-glycine (FG) Nups and bind RAN–GTP in the nucleus to release cargoes. Importin–RAN–GTP complexes then exit the nucleus; RAN–GTP hydrolysis and release of RAN–GDP in the cytoplasm then frees importins for the next round of import. Biportins and exportins (right) cooperatively bind export cargoes (light purple) and RAN–GTP in the nucleus. GTP hydrolysis of RAN in the cytosol releases export cargoes and RAN–GDP. Biportins are free for import, whereas unliganded exportins return to the nucleus. b | Kaps use their HEAT repeats to bind cargoes. Kap-binding elements can be specific linear signals (nuclear localization signals and nuclear export signals) within the cargoes, linear signals within adaptor proteins (which then bind cargoes with linear signals), folded domains or the combination of both linear and folded domains. Kaps display various charged and hydrophobic surfaces and their HEAT repeats are also arranged into diverse shapes that undergo different conformational changes, all allowing engagement of many diverse cargoes. c | RAN–GTP recognition is achieved by contacts made between RAN–GTP-specific switch 1, switch 2 and the basic patch with various HEAT repeats in the concave surface of all Kaps. HEAT repeats 1–4 are used by all Kaps, whereas other HEAT repeats are additionally used only by specific Kaps. Some Kaps also use long loops between or within HEAT repeats to contact switch 1 and the basic patch of RAN–GTP (not shown). d | Import: Kap binding to RAN–GTP and import cargoes is mutually exclusive, as a result of overlapping binding sites and steric hindrance and/or conformational changes in the superhelix of Kap that favour binding to RAN–GTP or cargo. Export: Kaps bind cargo and RAN–GTP cooperatively; RAN–GTP–cargo contacts and Kap conformational changes stabilize the ternary export complexes.
