FIGURE 4.
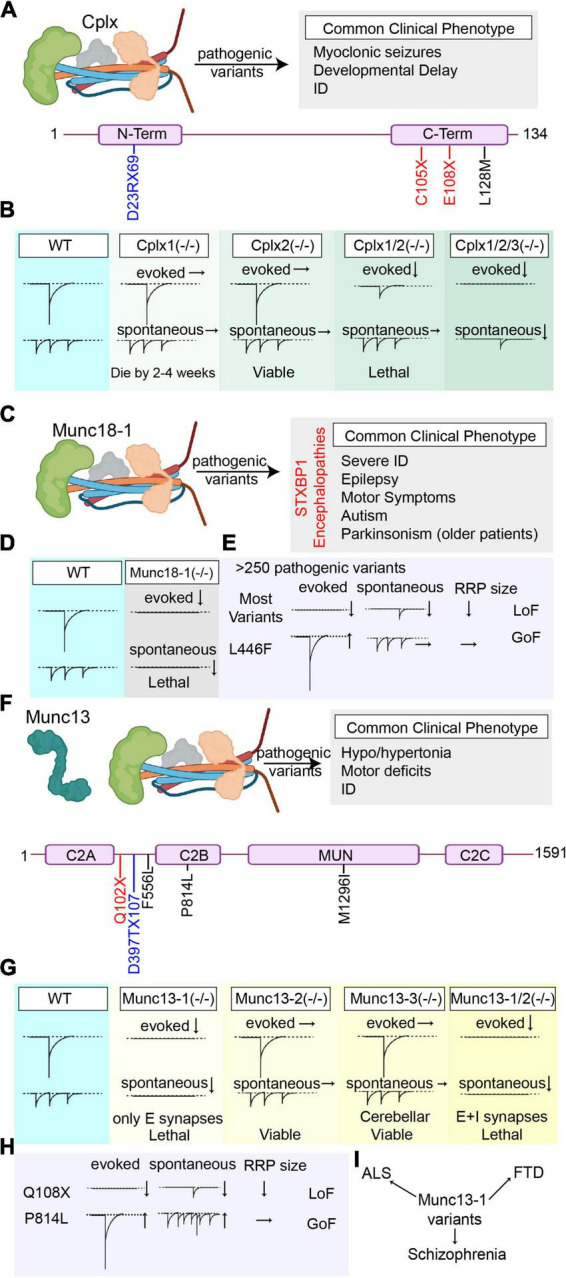
Overview of the pathogenic Cplx1, Munc18-1, and Munc13 variants’ effects on synaptic vesicle release. (A) Summary of all reported pathogenic Cplx1 (NCBI Accession #: NP_006642.1) variants and the most common clinical phenotype of the patients harboring these variants (Variants are color-coded based on their effects on the protein. Nonsense mutations are presented in red, missense mutations are presented in black, and frameshift mutations are presented in blue). (B) Graphical depiction demonstrating the changes in synaptic vesicle release upon genetic deletion of Cplx1 and/or Cplx 2 and upon triple knockout of Cplx1/2/3. (C) Summary of the most common clinical phenotype of the patients harboring pathogenic Munc18-1 (NCBI Accession #: NP_001361240.1) variants (STXBP1 encephalopathies). (D) Graphical depiction demonstrating the changes in synaptic vesicle release upon genetic deletion of Munc18-1. (E) Graphical summary of the findings explaining the loss-of-function outcomes of most Munc18-1 variants and the gain-of-function effects of L446F variant. (F) Summary of all reported pathogenic Munc13 (NCBI Accession #: AAC19406.1) variants and the most common clinical phenotype of the patients harboring these variants (Variants are color-coded based on their effects on the protein. Nonsense mutations are presented in red, missense mutations are presented in black, and frameshift mutations are presented in blue). (G) Graphical depiction demonstrating the changes in synaptic vesicle release upon genetic deletion of Munc13-1, Munc13-2, Munc13-3, and double knockout of Munc13-1/2. (H) Graphical summary of the findings explaining the effects of different Munc13 variants on different modes of neurotransmitter release (Q108X and P814L). (I) Munc13 variants are not only associated with early-onset neurobehavioral symptoms as the pathogenic variants in the other components of the release machinery, but also are associated with some complex diseases like ALS, FTS, and schizophrenia that develop later in life.
