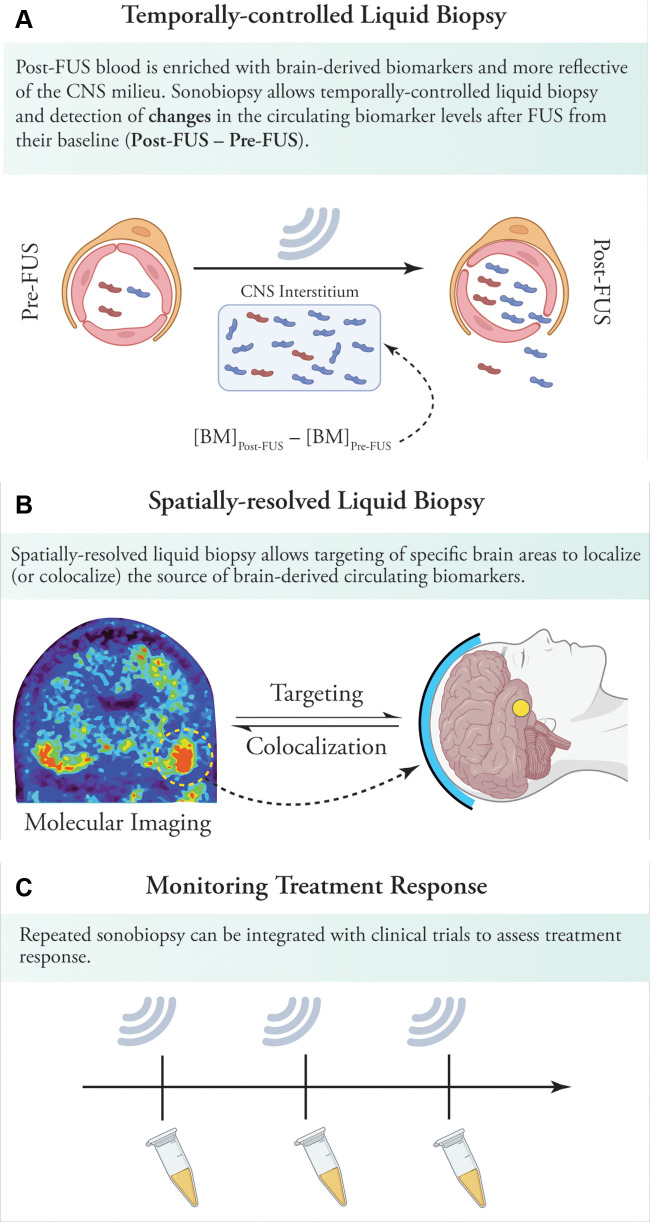
Figure 4:
Implications of sonobiopsy in clinical settings. (A) By opening the blood-brain barrier (BBB) and releasing proteins from the brain into the circulation, sonobiopsy can enrich plasma with brain-derived pathologic protein species. By simply collecting blood samples before and after sonications, sonobiopsy enables temporally controlled liquid biopsy and detection of changes in circulating pathologic protein species and other biomarkers after focused ultrasound. (B) Sonobiopsy can allow spatially resolved liquid biopsy. This can, in turn, be used for targeted BBB opening in brain areas with high protein deposits on molecular imaging (eg, tau or amyloid PET imaging). Sonobiopsy of these brain areas could shed light on the dominant pathogenic subtypes in heavily involved brain areas. In addition, the release of abnormal specific protein species can be colocalized to the brain regions showing the greatest metabolic, structural, or microstructural deficits. This can help identify the main culprits in pathogenesis of various neurodegenerative disorders. (C) Sonobiopsy can be readily integrated into focused ultrasound–induced BBB opening clinical trials to allow monitoring of treatment response by assessing changes in brain-derived pathogenic proteins. BBB = blood-brain barrier, BM = biomarker concentration in plasma, CNS = central nervous system, FUS = focused ultrasound.