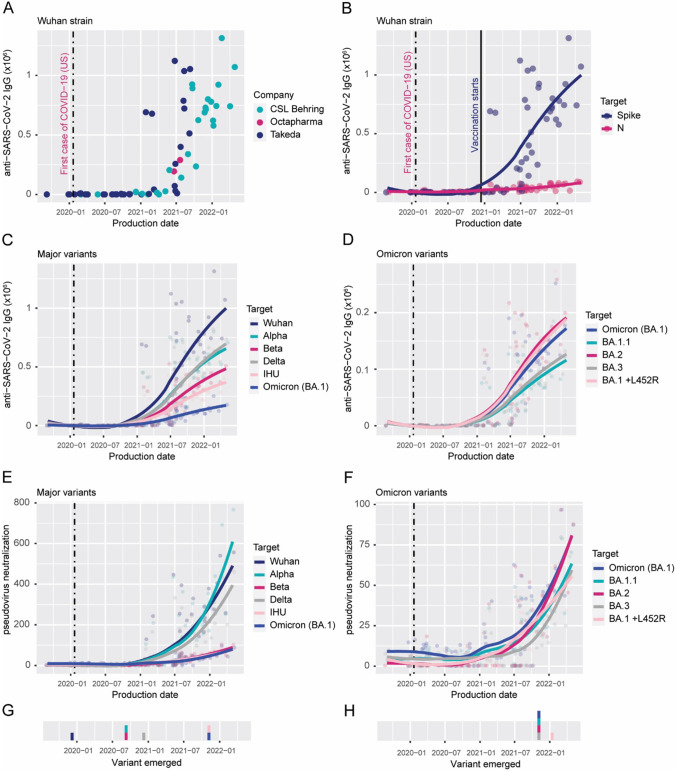
Fig. 1.
Vaccine-induced SARS-CoV-2 antibodies are consistently present in immunoglobulin preparations produced 18 months after the outbreak and later. A Anti-SARS-CoV-2 spike protein antibody quantity in batches of commercial immunoglobulin products used in clinical practice at the Immunodeficiency Unit at Karolinska University Hospital in relation to production date is plotted. The manufacturers are represented by different colors. B Antibody reactivity to the SARS-CoV-2 nucleocapsid (N) is specific for immunity after infection and rises slowly in comparison with the increase in antibody reactivity to the spike protein. Immunity in plasma donors is therefore mainly the result of vaccination. Antibody reactivity (C, D) and pseudovirus neutralization (E, F) to major virus variants and Omicron subvariants increase in parallel with reactivity to the original (Wuhan) strain. Lines were fitted using locally estimated scatterplot smoothing (LOESS) based on the individual immunoglobulin batch results (filled circles). Filled circles and lines are color-coded to show the results for each SARS-CoV-2 variant. Reactivity to certain VOCs is present in Ig batches produced before the virus variant was identified (G, H), implying that a degree of cross-reactivity exists between the tested variants. The virus variants are presented from top to bottom in the order they emerged (data from www.ecdc.europa.eu). A vertical dashed line indicates the first identified case of COVID-19 in the USA