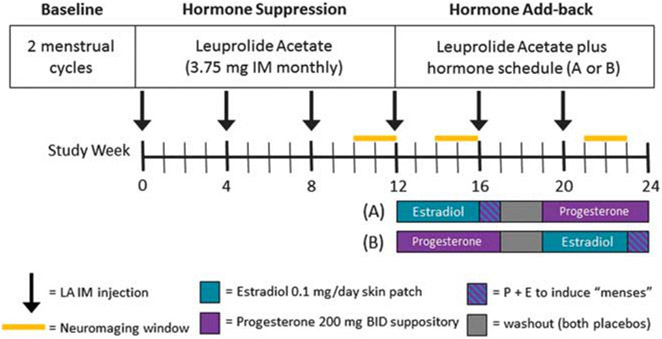
Figure 1.
Schematic diagram of GnRH agonist-induced hypogonadism and gonadal steroid replacement. Following a 2-month baseline evaluation period, women received 3.75 mg of Lupron (leuprolide acetate, purchased from TAP Pharmaceuticals, Chicago, IL, USA) by intramuscular injection every 4 weeks for 6 months. Lupron alone was administered for the first 12 weeks. After the Lupron-alone period, women received, in addition to Lupron, 17β estradiol (0.1 mg per day) by skin patch or progesterone suppositories (200 mg BID) for 5 weeks each. Women then were crossed-over to the alternative treatment (in a double blind, counterbalanced design). During the fifth week of estradiol add-back, progesterone suppositories (200 mg twice daily) were added to provide progesterone withdrawal-induced shedding of the endometrium and menses in order to prevent prolonged exposure of the endometrium to unopposed estrogen. The two replacement regimens were separated by a 2-week washout period. Three PET and three fMRI sessions were acquired: during Lupron alone, estradiol add-back and progesterone add-back periods. fMRI, functional magnetic resonance imaging; PET, positron emission tomography.