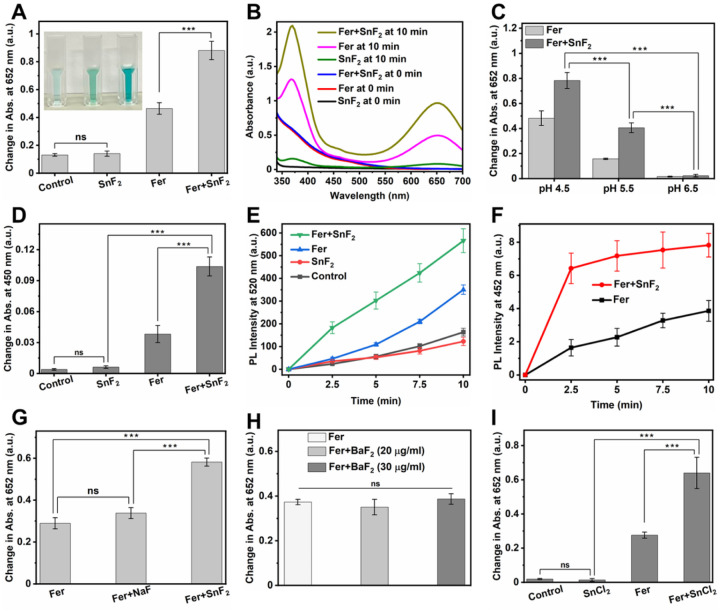
Figure 4. Enhanced catalytic activity of Fer in the presence of SnF2.
(A) Change in the absorption of TMB (chromogenic substrate) at 652 nm in different conditions. Inset: Photographs of TMB incubated with various reagents (left: SnF2 alone, middle: Fer alone, and right: Fer+SnF2) 10 min after H2O2 addition. (B) UV-visible absorption spectra of TMB in the presence of SnF2, Fer, or Fer+SnF2 at the times indicated. (C) Peroxidase-like activity of Fer and Fer+SnF2 at three pH values (4.5, 5.5, and 6.5) as determined by the colorimetric assay using TMB. (D) Change in the absorption of OPD at 450 nm in different conditions. The increase in absorption at 450 nm shows ROS production. (E) Comparison of change in PL intensities of DCF at 520 nm at various conditions. The increase in PL intensity at 520 nm depicts ROS production. (F) The change in PL intensity of 7-hydroxycoumarin at 452 nm as a function of time in the presence Fer with or without SnF2. The increase in the PL intensity at 452 shows the generation of •OH. (G to I) Effect of NaF (G), BaF2 (H), and SnCl2 (I) on the catalytic activity of Fer in 0.1 M sodium acetate buffer (pH 4.5). The data are presented as mean ± standard deviation. ***p < 0.001; ns, nonsignificant; one-way ANOVA followed by Tukey test.