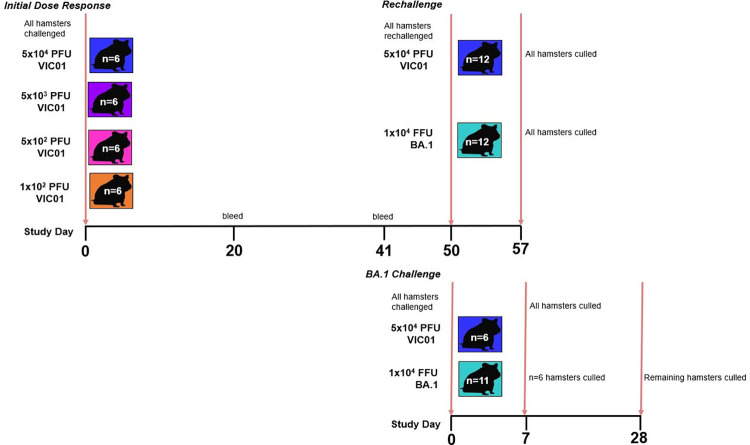
Fig 1. Study Design.
The study was split into three individual sections. Initial Dose Response: Hamsters (n = 6 per group with an equal male/female split) were infected intranasally with VIC01 at four different target doses: 5E+04, 5E+03, 5E+02 and 1E+02 PFU. Blood was collected from infected hamsters at baseline (day 0), 20- and 41-days post infection to assess humoral responses. Rechallenge: At 50 days following the initial VIC01 challenge, a rechallenge with either VIC01 or BA.1 was performed on 3 animals from each VIC01 convalescent group. This equalled a n = 12 in each rechallenge group with an equal male/female split. BA.1 Infection: Two naïve control groups were included in this study with the purpose of being run alongside the rechallenge; n = 6 hamsters were infected with VIC01 (equal male/female split) and n = 11 (3 male/8 female) hamsters were infected with BA.1. All animals were culled 7 days later, except for n = 5 (all female) in the BA.1 control group which were culled at 28 days post infection. The throats of all hamsters were swabbed to monitor viral shedding post-infection and rechallenge. At necropsy animals underwent terminal exanguination bled. Nasal cavity and lungs were collected for pathological examination. In addition, lung was collected to assess viral burden and spleen was collected to assess cellular immune responses.