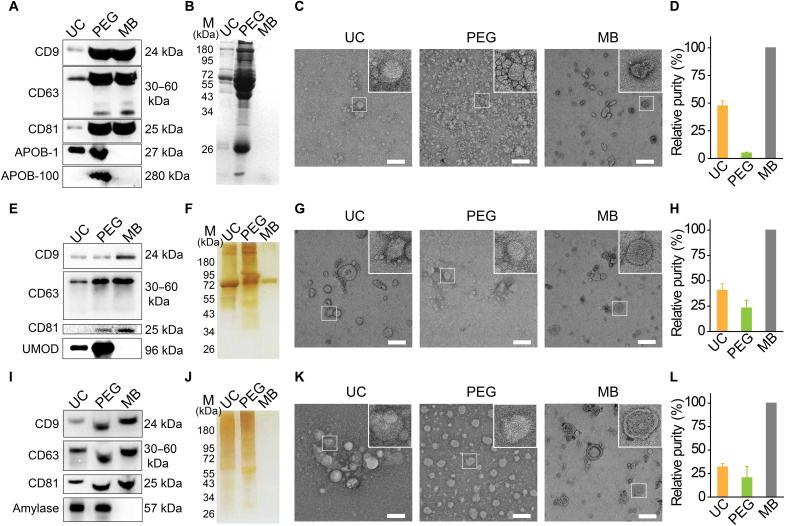
Fig. 5. Comparison of MB (denote as MB@CP-iPr here) and other methods for isolating EVs from various body fluids.
(A) Equal-sample-volume (2 μl of EV sample isolated from 300 μl of serum) Western blotting analysis of three common EV proteins (CD9, CD63, and CD81) in the same serum samples prepared by UC, PEG, and MB, respectively. APOB-1 and APOB-100 represent the most substantial contaminant reference of serum-EV’s purity. (B) SDS-PAGE analysis of protein components, (C) TEM images, and (D) relative purity of EVs isolated from the same human serum samples using the three different isolation methods. (E) Equal-sample-volume (5 μl of EV sample isolated from 10 ml of urine) Western blotting analysis of CD9, CD63, and CD81 in the same urine samples prepared by UC, PEG, and MB, respectively. UMOD is the most substantial contaminant reference of urine-EV’s purity. (F) SDS-PAGE analysis of protein components, (G) TEM images, and (H) relative purity of EVs isolated from the same human urine sample using the three different isolation methods. (I) Equal-sample-volume (5 μl of EVs sample isolated from 1 ml of saliva) Western blotting analysis of CD9, CD63, and CD81 in the same saliva samples prepared by UC, PEG, and MB, respectively. Amylase is the most substantial contaminant reference of saliva-EV’s purity. (J) SDS-PAGE analysis of protein components, (K) TEM images, and (L) relative purity of EVs isolated from the same human saliva sample using the three different isolation methods. Scale bars, 200 nm. The relative purity is presented in the bar charts (n = 3 independent experiments) where the enrichment purity of MB is set at 100%.