Figure 1.
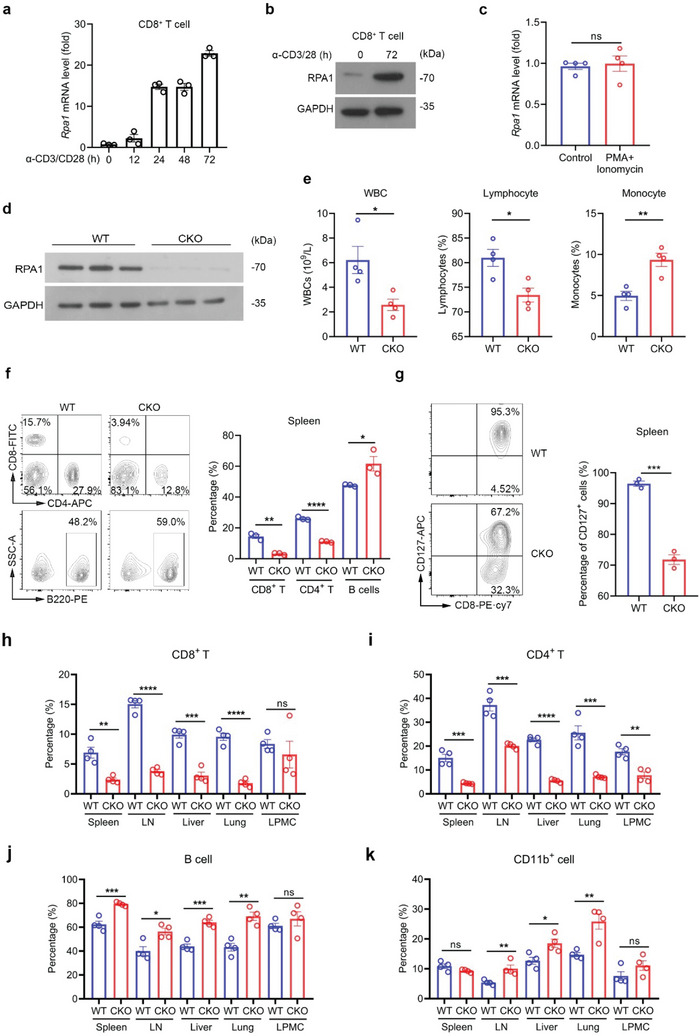
RPA1 is critical for dynamic homeostasis of peripheral T cell pool. a) Quantitative real‐time PCR (RT‐qPCR) analysis of Rpa1 mRNA level in naïve CD8+ T lymphocytes derived from 6‐week‐old wild‐type (WT) mice spleen. Antibodies against CD3 (2 µg mL−1) and CD28 (1 µg mL−1) were used to activate naïve T cells (n = 3 biological replicates, mean ± s.e.m.). The primers used for RT‐qPCR have been deposited in Table S1 (Supporting Information). b) Immunoblot analysis of protein level of RPA1 in naïve CD8+ T cells derived from 6‐week‐old WT mice spleen with or without anti‐CD3/CD28 antibody treatment. c) RT‐qPCR analysis of Rpa1 mRNA level in naïve CD8+ T cells derived from 6‐week‐old WT mice spleen. PMA (100 ng mL−1) and ionomycin (500 ng mL−1) were used to stimulate T cell activation (n = 4 biological replicates, mean ± s.e.m., ns, not significant (P > 0.05), two‐tailed unpaired Student's t‐test). The primers used for RT‐qPCR have been deposited in Table S1 (Supporting Information). d) Immunoblot analysis of protein level of RPA1 in T cells derived from 6‐week‐old WT and RPA1 conditional knockout (CKO) mice spleen (n = 3). e) Quantities of white blood cells (WBCs), lymphocytes and monocytes in 6‐week‐old male WT and CKO mice were analyzed by routine blood test using a HEMAVET 950FS Veterinary Multi‐species Hematology System (n = 4 biological replicates, mean ± s.e.m., *P < 0.05, **P = 0.0041, two‐tailed unpaired Student's t‐test). f) Flow cytometric analysis of the frequencies of CD4+ T cell, CD8+ T cell and B cell subsets in spleen from 6‐week‐old male WT and CKO mice (n = 3 mice, mean ± s.e.m., *P = 0.0409, **P = 0.0010, ****P < 0.0001, two‐tailed unpaired Student's t‐test). g) Flow cytometric analysis of CD127+ cells frequency in CD8+ T cells derived from 6‐week‐old male WT and CKO mice spleen (n = 3 mice, mean ± s.e.m., ***P = 0.0002, two‐tailed unpaired Student's t‐test). h–k) Flow cytometric analysis of the frequencies of CD8+ T cells (n = 4 mice, mean ± s.e.m., ns, not significant (P > 0.05), **P = 0.0030, ***P = 0.0002, ****P < 0.0001, two‐tailed unpaired Student's t‐test) h), CD4+ T cells (n = 4 mice, mean ± s.e.m., **P = 0.0018, ***P < 0.001, ****P < 0.0001, two‐tailed unpaired Student's t‐test) i), B cells (n = 4 mice, mean ± s.e.m., ns, not significant (P > 0.05), *P = 0.0119, **P = 0.0014, ***P < 0.001, two‐tailed unpaired Student's t‐test) j) and CD11b+ cells (n = 4 mice, mean ± s.e.m., ns, not significant (P > 0.05), *P = 0.0179, **P < 0.01, two‐tailed unpaired Student's t‐test) k) in spleen, lymph node (LN), liver, lung and lamina propria mononuclear cell (LPMC) from 6‐week‐old male WT and CKO mice.
