Figure 4.
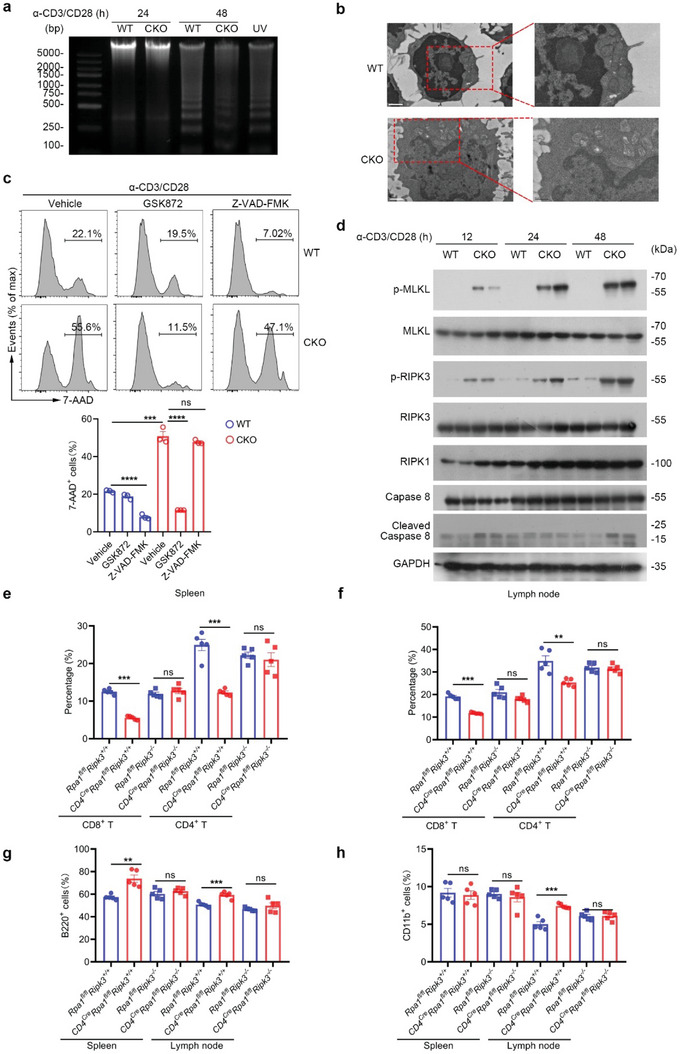
Loss of RPA1 causes peripheral T cell necroptosis post TCR engagement. a) Splenic CD8+ T cells derived from 6‐week‐old male WT and CKO mice stimulated with anti‐CD3 (2 µg mL−1) and anti‐CD28 (1 µg mL−1) antibodies for indicated time were harvested and prepared for the DNA fragmentation ladder assay. Naïve T cell from WT mice exposed to UV for 15 min was used as positive control. b) Representative pictures of CD8+ T cells derived from 6‐week‐old male WT and CKO mice spleen with anti‐CD3 (2 µg mL−1) and anti‐CD28 (1 µg mL−1) antibodies stimulation for 20 hours obtained from transmission electron microscopy (scale bars represent 1 µm). c) Splenic CD8+ T cells derived from 6‐week‐old male WT and CKO mice were stimulated with anti‐CD3 (2 µg mL−1) and anti‐CD28 (1 µg mL−1) antibodies for 24 h with or without cell death inhibitors. GSK872, 20 × 10−6 m; Z‐VAD‐FMK, 25 × 10−6 m. Cell viability was analyzed by 7‐AAD staining (n = 3 biological replicates, mean ± s.e.m., ns, not significant (P > 0.05), ***P = 0.0003, ****P < 0.0001, two‐tailed unpaired Student's t‐test). d) Immunoblot analysis of protein levels of phosphorylated MLKL, phosphorylated RIPK3, RIPK1 and cleaved caspase 8 in CD8+ T cells derived from 6‐week‐old WT and CKO mice spleen stimulated with anti‐CD3/CD28 antibodies for indicated times (n = 2 biological replicates). e,f) Flow cytometric analysis of the frequencies of indicated T cell subsets in spleen e) or lymph node (LN) f) from WT or CKO mice (n = 5 biological replicates, mean ± s.e.m., ns, not significant (P > 0.05), **P = 0.0035, ***P < 0.001, two‐tailed unpaired Student's t‐test). g,h) Flow cytometric analysis of the frequencies of B cells g) or CD11b+ myeloid cells h) in lymph node (LN) or spleen from WT or CKO mice (n = 5 biological replicates, mean ± s.e.m., ns, not significant (P > 0.05), **P = 0.0014, ***P < 0.001, two‐tailed unpaired Student's t‐test).
