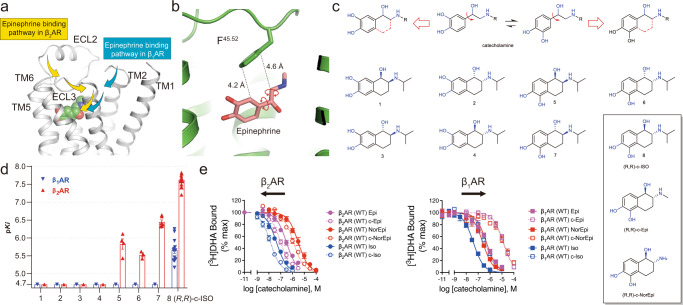
Fig. 1. Design and synthesis of conformationally-constrained catecholamine.
a Epi favors different binding pathways to enter the orthosteric pockets in the β1AR and β2AR7. b There is space between Epi and F45.52 to allow the receptor to accommodate for the conformational restriction of the catecholamine (PDB: 4LDO). c The design and synthesis flow of eight possible conformationally-constrained isoprenaline isomers and the chemical structures of (R,R)-c-Epi and (R,R)-c-NorEpi. d The (R,R)-isomer of c-ISO showed the highest affinity to both the β1AR and β2AR among all the eight possible isomers. Data were given as mean ± SEM of n = 3 (for 1-4,6), n = 4 (for 5), n = 5 (β1AR for 7), n = 6 (β2AR for 7), n = 13 (β2AR for 8), and n = 14 (β1AR for 8) independent experiments. e c-ISO, c-Epi, and c-NorEpi showed β2AR selectivity in a radioligand competition binding assay. Data were given as mean ± SEM of n = 3 (β1AR for Epi), n = 3(β2AR for Epi), n = 3 (β1AR for NorEpi), n = 5(β2AR for NorEpi), n = 3 (β1AR for ISO), n = 3(β2AR for ISO), n = 6 (β1AR for c-Epi), n = 6 (β2AR for c-Epi), n = 5 (β1AR for c-NorEpi), n = 5(β2AR for c-NorEpi), n = 3 (β1AR for cISO), n = 3(β2AR for cISO) independent experiments. Source data are provided as a Source Data file.