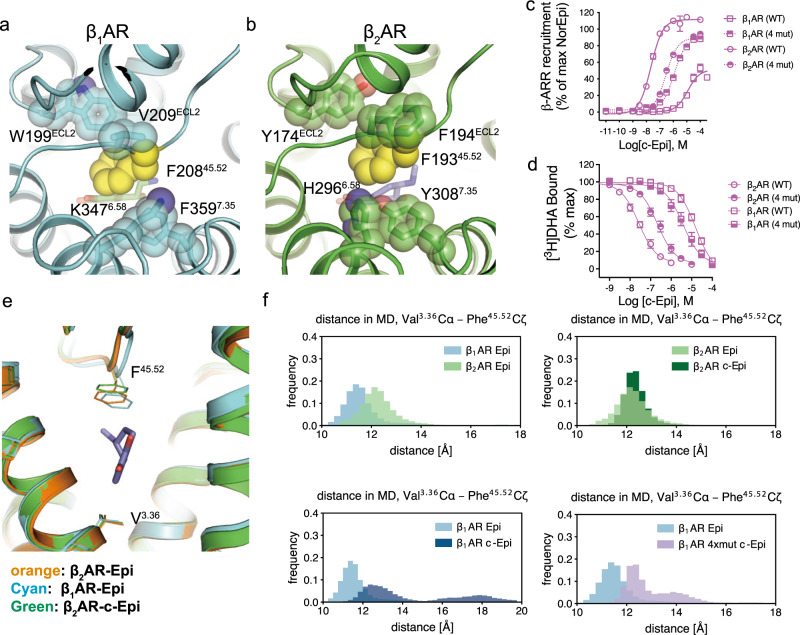
Fig. 4. Mutating four residues around the F45.52 affect c-Epi affinities in the β1AR and β2AR.
a, b F45.52 (yellow spheres) is conserved in the β1AR and β2AR, but is surrounded by differing residues. The different surrounding residues are W199ECL2, V209ECL2, K3476.58, and F3597.35 in the β1AR (a) and Y174ECL2, F194ECL2, H2966.58, and Y3087.35 in the β2AR (b). c, d Mutating the four residues surrounding F45.52 reduced c-Epi affinity to the β2AR and increased c-Epi affinity to the β1AR, in a β-arrestin recruitment assay (c) and in a competition binding assay (d). Data were given as mean ± SEM of n = 7 (β1AR (4 mut), β2AR (4 mut), arrestin), n = 15 (β1AR, arrestin), and n = 21 (β2AR, arrestin), n = 6 (β1AR, β2AR, β1AR (4 mut), β2AR (4 mut),DHA binding) independent experiments. Source data are provided as a Source data file. e F45.52 has a slightly different conformation in the β2AR-c-Epi, β2AR-Epi, and β1AR-Epi structures, and as a result, the distance between V3.36 and F45.52 is longer in the β2AR-c-Epi structure than in the β2AR-Epi structure, while the distance is the shortest in the β1AR-Epi structure. f The distribution for the distance between the Cα atom of V3.36 and the Cζ atom of F45.52 in MD simulations are displayed for the different simulation conditions. The distribution is shown in light blue for β1AR-Epi, dark blue for β1AR-c-Epi, light green for β2AR-Epi, dark green for β2AR-c-Epi and purple for β1AR_4mut-c-Epi. Cζ of F45.52 is located deeper in the pocket for β1AR-Epi compared to β2AR-Epi, and β1AR-c-Epi shows a similar distribution as β1AR-Epi. The deeper position in β1AR cannot accommodate for c-Epi and leads to a distortion of the F45.52 conformation and, therefore, to higher distances. For β1AR_4mut-c-Epi, distances at a β2AR-like level are observed, suggesting that the β2AR-like surrounding of F45.52 leads to conformations of F45.52 that are located less deep in the pocket as for β1AR-Epi.