Highlights
-
•
Chronotropic incompetence was more frequent in obese adolescents compared to their lean counterparts.
-
•
C-reactive protein concentrations were increased in obese adolescents with chronotropic incompetence.
-
•
The C-reactive protein and total cholesterol concentration were independently associated with the maximal chronotropic index.
-
•
Chronotropic incompetence within obese adolescents was related to exercise intolerance.
Keywords: Adolescents, Cardiometabolic health, Exercise tolerance, Heart rate, Obesity
Abstract
Background
Adults with obesity may display disturbed cardiac chronotropic responses during cardiopulmonary exercise testing, which relates to poor cardiometabolic health and an increased risk for adverse cardiovascular events. It is unknown whether cardiac chronotropic incompetence (CI) during maximal exercise is already present in obese adolescents and, if so, how that relates to cardiometabolic health.
Methods
Sixty-nine obese adolescents (body mass index standard deviation score = 2.23 ± 0.32, age = 14.1 ± 1.2 years; mean ± SD) and 29 lean adolescents (body mass index standard deviation score = –0.16 ± 0.84, age = 14.0 ± 1.5 years) performed a maximal cardiopulmonary exercise testing from which indicators for peak performance were determined. The resting heart rate and peak heart rate were used to calculate the maximal chronotropic response index. Biochemistry (lipid profile, glycemic control, inflammation, and leptin) was studied in fasted blood samples and during an oral glucose tolerance test within obese adolescents. Regression analyses were applied to examine associations between the presence of CI and blood or exercise capacity parameters, respectively, within obese adolescents.
Results
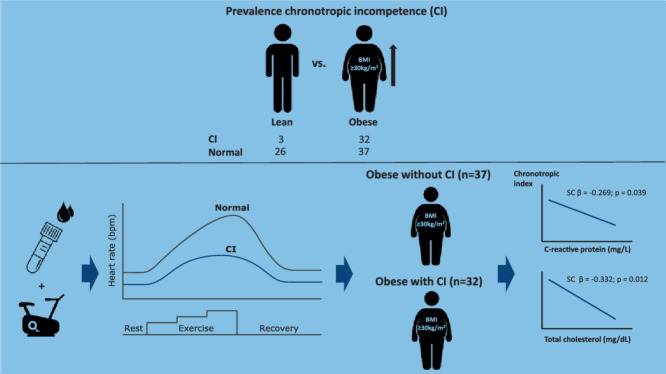
CI was prevalent in 32 out of 69 obese adolescents (46%) and 3 out of 29 lean adolescents (10%). C-reactive protein was significantly higher in obese adolescents with CI compared to obese adolescents without CI (p = 0.012). Furthermore, peak oxygen uptake and peak cycling power output were significantly reduced (p < 0.05) in obese adolescents with CI vs. obese adolescents without CI. The chronotropic index was independently related to blood total cholesterol (standardized coefficient β = –0.332; p = 0.012) and C-reactive protein concentration (standardized coefficient β = –0.269; p = 0.039).
Conclusion
CI is more common in the current cohort of obese adolescents, and is related to systemic inflammation and exercise intolerance.
Graphical Abstract
1. Introduction
The prevalence of obesity has increased considerably among adolescents over the past several decades to become one of the most significant health concerns worldwide.1 Recent data indicate that, globally, the number of children and adolescents with obesity increased to 62 million by 2016.1 Obese adolescents face major health and socio-economic impacts,2,3 which include being at greater risk for developing endothelial dysfunction, hypertension, insulin resistance, nonalcoholic fatty liver disease, respiratory and orthopedic disorders, or for suffering from psychosocial difficulties, even into adulthood.4, 5, 6 In addition to the medical complications related to obesity in adolescents, suboptimal physiological responses to exercise have also been observed;7 in particular, a reduced peak cycling power output7, 8, 9 and peak oxygen uptake/kg lean tissue mass. 7
In adults with obesity (those with or without type 2 diabetes mellitus), chronotropic incompetence (CI) is often observed.10, 11, 12 CI is defined as the inability of the heart rate (HR) to increase in accordance with the metabolic demand, leading to a lowered peak HR (HRpeak) (<80% of maximal predicted HRpeak).13,14 It has been shown that both peak and resting HR account for over forty percent of the variability explaining exercise capacity, and that this kind of modification in cardiac behavior leads to reductions in exercise capacity.12
In adult individuals who are obese, CI is independently related to an elevated risk for major adverse cardiovascular events (e.g., acute myocardial infarction or heart failure) and premature death.15,16 There are several mechanisms that could potentially account for the association of obesity and CI, including a blunted catecholamine response, an abnormal baroreflex function, or a decreased catecholamine sensitivity and beta-adrenergic receptor density, all of which ultimately lead to autonomic dysfunction.17,18 This supports the concept that abnormalities in autonomic balance may precede manifestations of cardiovascular disease and may contribute to the early identification of persons at high risk for sudden death.16
Despite the well-established association between CI and prognosis of all-cause mortality in adults with obesity,15,16 the prevalence of CI in adolescents with obesity has not been investigated, nor has its association with cardiometabolic health or exercise tolerance. These are important questions to answer, however, as they may affect the methodology for prescribing/monitoring exercise intensity based on HR and lead us to a deeper understanding of how adolescent obesity may affect cardiometabolic health and exercise performance.
Therefore, this study aims to (1) examine the prevalence of CI in adolescents with obesity and (2) understand whether CI is related to cardiometabolic health and exercise tolerance in obese adolescents. Based on literature from studies involving obese adults,10,12 we hypothesize that CI will be more prevalent in obese vs. lean adolescents, and that CI relates to a worse cardiometabolic health and exercise performance.
2. Methods
The study was carried out according to an observational, cross-sectional design and was performed at Jessa Hospital (Hasselt, Belgium), as described previously.19 From midnight prior to a 1-day hospitalization, all subjects refrained from consuming food (with the exception of water ad libitum) to prevent short-term metabolic effects on outcome parameters. After registration of general characteristics, anthropometry, body composition, Tanner stage, and blood pressure were measured. In addition, venous blood samples were collected (in a fasted state), and an oral glucose tolerance test (OGTT) was performed to examine participants’ cardiometabolic health. One hour prior to cardiopulmonary exercise testing (CPET), a standardized meal (total energy = 296 kcal; composed of 3 g fats, 56 g carbohydrates, and 9 g proteins) was consumed.
2.1. Subjects
Adolescents with obesity were recruited from the pediatric clinic of Jessa Hospital. Data from lean adolescents were taken from a previous study performed by our research group and used only to determine the CI prevalence.19 Participants were between 11 and 17 years of age and free from any known chronic cardiovascular, renal, pulmonary or orthopedic disease. The International Obesity Task Force criteria and body fat percentage (>95th percentile) were used to categorize the participants into a lean group and an obese group.20,21 Sixty-nine obese adolescents (body mass index (BMI) standard deviation score (SDS = 2.23 ± 0.32, age = 14.1 ± 1.2 years; mean ± SD) and 29 lean adolescents (BMI SDS = –0.16 ± 0.84, age = 14.0 ± 1.5 years) were included into the study. All participants and their parents/legal guardians received oral and written information about the aim and protocol of the study and gave their written informed consent prior to participation. The study protocol was approved by the Medical Ethical Committee of the Jessa Hospital and Hasselt University (Hasselt, Belgium) and was performed according to the Declaration of Helsinki (2013). The present study is registered at clinicaltrials.gov as NCT04185753.
2.2. Auxological parameters
Body height was measured to the nearest 0.1 cm using a wall-mounted Harpenden stadiometer (ICD 250 DW, De Grood Metaaltechniek, Nijmegen, the Netherlands) with participants barefoot. Body weight was determined to the nearest 0.1 kg using a digital-balanced weighing scale (Seca 770; Seca GmbH, Hamburg, Germany) with participants in underwear. The BMI was calculated from weight and height measurements (weight/height²). The body height and BMI SDS were calculated using the LMS method, as described by Cole et al.:20,22 body height-SDS = (body height/M)L − 1)/(L × S) and BMI-SDS = (BMI/M)L − 1)/(L × S), where M is the median BMI by age, S the coefficient of variation of BMI, and L expresses the skewness of the BMI distribution in terms of the Box-Cox power needed to transform the data to near normality.22 Waist and hip circumferences were measured in triplicate to the nearest 0.1 cm using the flexible metric measuring tape Seca 201 (Seca GmbH), with participants in standing position, barefoot and in underwear. Waist circumference was measured at the midpoint between the lower rib margin and the top of the iliac crest. Hip circumference was measured at the widest circumference of the hip at the level of the greater trochanter. Waist-to-hip ratio was calculated by dividing waist circumference (cm) by hip circumference (cm). Body composition was evaluated by tetrapolar bioimpedance measurement using the Bodystat 1500 MDD® monitoring unit (EuroMedix, Leuven, Belgium) with subjects in supine position.23 Electrodes were placed on the dorsal surface of the wrist and hand (just behind the middle finger) and the ankle and foot (just behind the middle toe) according to manufacturer's instructions. Body fat percentage was calculated using the formula of Houtkooper.24
2.3. Blood pressure, pubertal development stage, and physical activity evaluation
Blood pressure was measured in supine position using an electronic sphygmomanometer (Omron, Omron Healthcare, Lake Forest, IL, USA) after a resting period of 5 min. Pubertal status was assessed using Tanner's scale, which defines physical features of development according to external primary and secondary sex characteristics; the assessment relied on observations by a pediatrician as well as on the adolescents’ own opinions based on a figure.25,26 Level of physical activity was determined using the validated Dutch Physical Activity Questionnaires for Adolescents (PAQ-A).27
2.4. Biochemical analysis
Venous blood samples were taken from participants for the measurement of blood parameters. Plasma glucose, lipid profile (blood total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein cholesterol, and triglyceride concentrations), C-reactive protein (CRP), and serum insulin concentrations were automatically assessed on Roche Cobas 8000 (Roche Diagnostics International Ltd., Rotkreuz, Switzerland). Blood glycated hemoglobin concentration was measured using ion exchange chromatography (Menarini HA-8180 hemoglobin concentration auto-analyzer, Menarini Diagnostics, Diegem, Belgium). Serum leptin concentrations were measured using radioimmunoassay (RIA; LINCO Research Inc., St. Louis, MO, USA). All coefficients of variation for these assays were less than 15%.
2.5. Insulin sensitivity and beta cell function
A standard 5-point OGTT was performed for assessment of whole-body/tissue-specific insulin sensitivity and beta cell function. Subjects ingested a solution (200 mL) containing 75 g dextrose, and venous blood samples were obtained at 0 min, 30 min, 60 min, 90 min, and 120 min for assessment of glucose and insulin concentration. Blood samples were automatically assessed on Roche Cobas 8000 (Roche Diagnostics International Ltd.). From glucose and insulin concentrations, homeostatic model assessment for insulin resistance was calculated by: fasting glucose (mg/dL) × fasting insulin (mU/L)/405.28 Tissue-specific insulin resistance was calculate using the hepatic insulin resistance index and the muscle insulin sensitivity index. The hepatic insulin resistance index was calculated as the product of the area under curve (AUC) for glucose and insulin during the first 30 min of the OGTT (glucose0–30(AUC in mg/dL h) × insulin0–30(AUC in mU/L h)), and the muscle insulin sensitivity index was calculated as the rate of decay of glucose concentration during the OGTT divided by the mean insulin concentration during the OGTT (in mg/dL/min/mU/L). The rate of decay was calculated as the slope of the least square fit to the decline in glucose concentration from peak to nadir, as described by Vogelzangs et al.29 In addition, whole-body insulin sensitivity index was calculated by: 10000/√(fasting glucose (mg/dL) × fasting insulin (mU/L)) × (mean glucose during OGTT (mg/dL) × mean insulin during OGTT (mU/L)).28 The quantitative insulin sensitivity check index (QUICKI) was calculated as: 1/log(fasting insulin (mU/L) + log(fasting glucose (mg/dl).28 Beta cell function was estimated by calculation of the insulinogenic index, that is, the ratio of increment of insulin (mU/L) and glucose (mg/dL) in the first 30 min of the OGTT.30 The AUC for glucose and insulin for the 2-h period was calculated using the trapezoidal rule.31
2.6. CPET
CPET was performed up to volitional exhaustion using an electronically braked cycle ergometer (eBike, GE Medical systems, Milwaukee, WI, USA) controlled by the Cardiosoft electrocardiography software (Cardiosoft 6.6, GE Medical systems, Freiburg, Germany). At the beginning of each test day, a gas and volume calibration was performed according to manufacturer's instructions. During the test, environmental temperature was kept stable between 19°C and 21°C. The exercise test (ramp protocol) included a 1-min pre-exercise resting period, a 1-min unloaded warm-up cycling phase, as well as an incremental exercise cycling period with an initial workload of 40 W and an increasing workload of 20 W per minute. During warm-up cycling and incremental exercise, a cycling frequency of 60–70 revolutions per minute (rpm) had to be maintained. The test was ended when the subject failed to maintain a pedal frequency of at least 60 rpm. All subjects were verbally encouraged during exercise testing to achieve maximal effort, measured by a respiratory gas exchange ratio of ≥1.05 and the subjective opinion of an experienced tester who confirmed whether or not a maximal exercise test was executed. The tester's expert opinion was based on subjective features described by Bongers et al.,32 including dyspnea, sweating, facial flushing, a clear unwillingness to continue, and a sustained drop in the participant's pedaling frequency from 60 rpm despite verbal encouragement. After cessation of exercise, workload was set at 45 W; subjects cycled for 2 min during active recovery with a cycling frequency of 50 rpm.
With the aid of continuous pulmonary gas exchange analysis (Jaeger MasterScreen CPX Metabolic Cart, CareFusion Germany GmbH, Hoechberg, Germany) oxygen uptake (V̇O2), carbon dioxide output (V̇CO2), minute ventilation (V̇E), equivalents for oxygen uptake (V̇E/V̇O2) and carbon dioxide production (V̇E/V̇CO2), tidal volume, breathing frequency, and the respiratory gas exchange ratio were determined breath by breath, and data were averaged every 10 s. Using a 12-lead electrocardiography device (KIS Multilead, GE Medical systems, Freiburg, Germany) HR was monitored and averaged every 10 s. From this parameter, oxygen pulse (V̇O2/HR) was calculated. Age-predicted maximal HR was calculated using the equation HR = 220 − age in years. Maximal chronotropic response index (CRImax) was calculated using the following equation: (actual HRpeak − HRrest)/(predicted HRpeak − HRrest).13 CI was defined as the inability to achieve a CRImax ≥ 0.80.13 The V̇O2 efficiency slope was calculated by a linear least square regression of V̇O2 on the logarithmic of V̇E using all exercise data.32 The first ventilatory threshold was determined using the V-slope method.33 The second ventilatory threshold was determined using the V̇E vs. V̇CO2 plot, on the point where V̇E increases out of proportion to V̇CO2.34 Exercise tolerance was assessed by the peak workload.
2.7. Statistical analysis
Statistical analysis was performed by SPSS Version 24.0 (IBM Corp., Armonk, NY, USA). Data were expressed as means ± SD. A Shapiro–Wilk test was used to test normality of the data (p < 0.05). Comparisons between groups were tested using the χ2 test for categorical variables. Differences between continuous variables of subject characteristics were assessed using a one-way analysis of variance with a Bonferroni post hoc comparison test for normally distributed data and the Kruskal–Wallis test (Dunn's post hoc comparison test) for non-normally distributed data. Differences between continuous variables (e.g., blood and CPET) were assessed using independent samples t tests for normally distributed data and Mann-Whitney U tests for abnormally distributed data within obese adolescents. A two-way repeated measures analysis of variance was used to assess whether there were differences in HR behavior during exercise testing between obese adolescents with CI and those without: an interaction effect was evaluated where group (obese vs. obese with CI) was a between-subjects factor, and time (percentage of V̇O2peak) was a within-subjects factor. A post hoc analysis (Bonferroni post hoc comparison test) was performed when the between-subjects factor was statistically significant. Multivariate linear regression analysis was applied to examine relations between the CRImax and variables of cardiometabolic health, corrected for age, sex, and Tanner stage. Variables with a beta-coefficient <0.1 were left out of consideration. A p value of <0.05 (2-tailed) was considered statistically significant.
The sample size was based on a previous study by Marinus et al.35 that showed obese adolescents had a significantly decreased HRpeak (192 ± 8 bpm vs. 183 ± 11 bpm).35 Based on the difference in HRpeak (effect size d = 0.96), a statistical power of >0.8, and a 2-sided α of 0.05, it was calculated that a sample size of 19 obese individuals with CI and 19 obese adolescents without CI must be included in the present study. In addition, a secondary outcome parameter with regard to systemic inflammation was included. Huang et al.36 found that hsCRP concentrations were significantly different between adults with and without CI. Using the same values as stated above and an effect size of 0.39, it was calculated that a sample size of 16 obese individuals with and 16 without CI must be included in the present study. Taking into account a drop-out rate of 10%, the number of participants needed to be raised to at least 21 obese adolescents with CI and 21 obese adolescents without CI, which resulted in a final sample size of 42 subjects.
3. Results
3.1. Subject characteristics
A total of 98 participants (69 obese and 29 lean adolescents) were eligible and completed the study. Due to previously undetected anemia, data from 1 lean adolescent was excluded. By design, body weight (p < 0.001), BMI (p < 0.001) and BMI-SDS (p < 0.001), waist circumference (p < 0.001), hip circumference (p < 0.001), waist-to-hip ratio (p < 0.001), percentage of body fat (p < 0.001), and systolic blood pressure (SBP) (p < 0.001) were elevated in obese vs. lean adolescents (Table 1). Compared with their lean counterparts, physical activity levels (PAQ-A score) were lower in obese adolescents with CI (p = 0.020) but similar in those without. Age, sex, body height, body height-SDS, diastolic blood pressure (DBP), and Tanner stage were comparable between groups (p > 0.05) (Table 1).
Table 1.
Subject characteristics of obese and lean individuals (n or mean ± SD).
| General features | Lean (n = 29) | Obese (n = 37) | Obese CI (n = 32) | p |
|---|---|---|---|---|
| Age (year) | 14.0 ± 1.50 | 14.1 ± 1.30 | 14.1 ± 1.10 | 0.382 |
| Sex (n) | 0.489 | |||
| Male | 16 | 21 | 15 | |
| Female | 13 | 16 | 17 | |
| Body weight (kg) | 54.7 ± 10.8* # | 91.9 ± 18.6 | 96.4 ± 21.0 | <0.001 |
| Body height (cm) | 166.8 ± 8.9 | 166.9 ± 9.5 | 166.6 ± 7.4 | 0.977 |
| Body height-SDS | 0.68 ± 1.00 | 0.72 ± 1.17 | 0.69 ± 1.05 | 0.979 |
| BMI (kg/m²) | 19.5 ± 2.4* # | 32.7 ± 4.4 | 34.6 ± 5.9 | <0.001 |
| BMI-SDS | –0.16 ± 0.84* # | 2.20 ± 0.31 | 2.27 ± 0.33 | <0.001 |
| Waist circumference (cm) | 67.4 ± 6.2* # | 101.5 ± 12.2 | 101.4 ± 12.0 | <0.001 |
| Hip circumference (cm) | 78.7 ± 8.3*# | 106.5 ± 8.8 | 107.0 ± 10.3 | <0.001 |
| Waist-to-hip ratio | 0.86 ± 0.11*# | 0.95 ± 0.07 | 0.95 ± 0.08 | <0.001 |
| Body fat (%) | 14.1 ± 7.6*# | 36.0 ± 5.2 | 39.2 ± 6.8 | <0.001 |
| PAQ-A score | 2.51 ± 0.50 # | 2.24 ± 0.66 | 2.04 ± 0.57 | 0.020 |
| SBP (mmHg) | 114 ± 10* | 137 ± 26 # | 124 ± 12 | <0.001 |
| DBP (mmHg) | 70 ± 7 | 75 ± 21 | 73 ± 11 | 0.393 |
| CI (n) | 29/3 | 0/37 | 32/32 | <0.001 |
| Development stage (n) | 0.759 | |||
| Tanner Stage 1 | 2 | 3 | 1 | |
| Tanner Stage 2 | 2 | 1 | 2 | |
| Tanner Stage 3 | 4 | 6 | 6 | |
| Tanner Stage 4 | 7 | 3 | 6 | |
| Tanner Stage 5 | 14 | 17 | 17 |
Notes: Comparisons between groups were tested using the χ2 test for categorical variables. For continuous variables, comparisons between groups were tested using a one-way analysis of variance (Bonferroni post hoc comparison test) for normally distributed data and the Kruskal-Wallis test (Dunn's post hoc comparison test) for non-normally distributed data. The p values reported represent the overall analysis of variance.
* p < 0.05, compared with obese group; #p < 0.05, compared with obese CI group.
Abbreviations: BMI = body mass index; BMI-SDS = body mass index standard deviation score; CI = chronotropic incompetence; DBP=Diastolic blood pressure; PAQ-A = Dutch Physical Activity Questionnaire for Adolescents; SBP = systolic blood pressure; SDS = standard deviation score.
3.2. Prevalence of CI in lean vs. obese adolescents
CI was present in 32 out of 69 (46%) obese adolescents and 3 out of 29 (10%) lean adolescents. These CI prevalence rates were significantly different between lean and obese adolescents (p < 0.01). No differences in clinical features were found between obese adolescents with and without CI, except for SBP (124 ± 12 mmHg vs. 137 ± 26 mmHg; p = 0.032).
3.3. CI and its relation to cardiometabolic health within obese adolescents
3.3.1. Blood parameters
Only CRP was significantly higher (p = 0.012) in obese adolescents with CI (4.9 ± 3.2 mg/L) than in obese adolescents without CI (2.2 ± 2.2 mg/L). With respect to glycemic control and lipid profile, no differences were found between obese adolescents with or without CI (p > 0.05) (Table 2).
Table 2.
Cardiometabolic risk factors and parameters regarding glycemic control in obese adolescents with and without chronotropic incompetence (mean ± SD).
| Obese (n = 37) | Obese CI (n = 32) | p | |
|---|---|---|---|
| Cardiovascular risk factors | |||
| C-reactive protein (mg/L) | 2.2 ± 2.2 | 4.9 ± 3.2 | 0.012 |
| Total cholesterol (mg/dL) | 152 ± 33 | 158 ± 27 | 0.388 |
| LDL cholesterol (mg/dL) | 85 ± 24 | 93 ± 25 | 0.282 |
| HDL cholesterol (mg/dL) | 44 ± 11 | 42 ± 10 | 0.497 |
| Triglycerides (mg/dL) | 112 ± 67 | 123 ± 75 | 0.550 |
| Triglyceride-to-HDL cholesterol ratio | 2.8 ± 2.0 | 3.3 ± 2.6 | 0.571 |
| Leptin (µg/L) | 46.9 ± 22.0 | 8.7 ± 6.3 | 0.703 |
| Glycemic control | |||
| Fasting glucose (mg/dL) | 91 ± 6 | 92 ± 6 | 0.636 |
| Fasting insulin (mU/L) | 24 ± 13 | 28 ± 5 | 0.096 |
| Glycated hemoglobin (%) | 5.4 ± 0.3 | 5.4 ± 0.2 | 0.313 |
| HOMA-IR | 5.4 ± 3.2 | 6.1 ± 3.7 | 0.116 |
| MISI (mg/dL/min/mU/L) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.710 |
| HIRI ((mg/dL) × h × (mU/lL) × h) | 51.2 ± 18.9 | 58.1 ± 21.0 | 0.179 |
| WBISI | 2.38 ± 1.22 | 1.95 ± 1.17 | 0.140 |
| QUICKI | 0.31 ± 0.03 | 0.30 ± 0.03 | 0.347 |
| IGI (mU/L/mg/dL) | 3.46 ± 1.93 | 4.96 ± 3.81 | 0.191 |
| AUC glucose ((mg/dL) × h) | 512 ± 86 | 484 ± 77 | 0.195 |
| AUC insulin ((mU/L) × h) | 702 ± 670 | 680 ± 438 | 0.883 |
Note: Comparisons between the 2 groups were performed using the independent-samples t test or Mann-Whitney U test.
Abbreviations: AUC = area under curve; HDL = high-density lipoprotein; HIRI = hepatic insulin resistance index; HOMA-IR = homeostatic model assessment of insulin resistance; IGI = insulinogenic index; LDL = low-density lipoprotein; MISI = muscle insulin sensitivity index; QUICKI = quantitative insulin sensitivity check index; WBISI = whole-body insulin sensitivity index.
3.3.2. Exercise tolerance and cardiopulmonary function
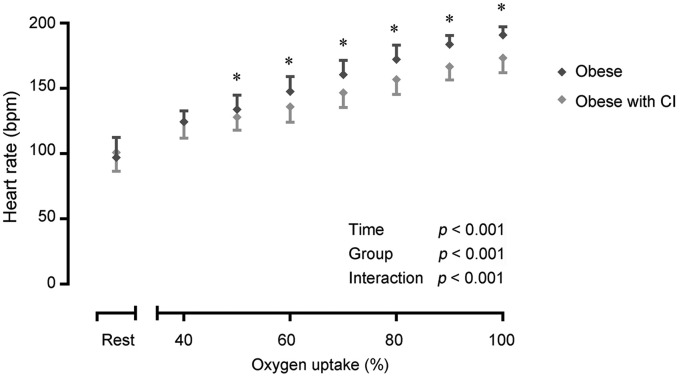
A reduction in the V̇O2peak (mL/min: p = 0.005; mL/min/kg: p < 0.001), V̇CO2peak (p < 0.001), V̇Epeak (p < 0.001), Vtpeak (p = 0.029), BFpeak (p = 0.022), oxygen uptake efficiency slope (p = 0.035), RERpeak (p = 0.001), peak workload (p < 0.001), and CRImax (p < 0.001) was observed in obese adolescents with CI vs. obese adolescents without CI (Table 3). Although the resting HR was comparable between both obese groups, time (p < 0.001), group (p < 0.001), and time × group interaction (p < 0.001) effects were found for the HR response during CPET (Fig. 1). The HR increase becomes significantly less steep at 50% of V̇O2peak in obese adolescents with CI, in comparison to obese adolescents without CI (50% V̇O2peak: p = 0.023; 60%–100% V̇O2peak: p < 0.001).
Table 3.
Cardiopulmonary function during cardiopulmonary exercise testing in obese adolescents with and without chronotropic incompetence (mean ± SD).
| Obese normal (n = 37) | Obese CI (n = 32) | p | |
|---|---|---|---|
| V̇O2peak (mL/min) | 2303 ± 459 | 1999 ± 414 | 0.005 |
| V̇O2peak per weight (mL/min/kg) | 25.5 ± 5.5 | 21.0 ± 3.6 | <0.001 |
| V̇O2peak (mL/min) | 2750 ± 517 | 2268 ± 458 | <0.001 |
| Peak minute ventilation (L/min) | 80 ± 17 | 64 ± 15 | <0.001 |
| Peak tidal volume (L) | 2.00 ± 0.51 | 1.75 ± 0.38 | 0.029 |
| Peak breathing frequency (breaths/min) | 41 ± 7 | 37 ± 7 | 0.022 |
| Oxygen uptake efficiency slope | 2492 ± 529 | 2232 ± 465 | 0.035 |
| Peak ventilatory equivalent V̇O2 | 33.6 ± 6.8 | 31.0 ± 5.0 | 0.078 |
| Peak ventilatory equivalent V̇CO2 | 28.6 ± 3.4 | 27.8 ± 2.8 | 0.264 |
| Peak respiratory exchange ratio | 1.20 ± 0.08 | 1.14 ± 0.07 | 0.001 |
| Peak oxygen pulse (mL/heart beat) | 12.1 ± 2.5 | 11.6 ± 2.5 | 0.305 |
| Peak workload (W) | 172 ± 31 | 137 ± 27 | <0.001 |
| Ventilatory threshold 1 (mL/min) | 1184 ± 231 | 1151 ± 231 | 0.552 |
| Ventilatory threshold 2 (mL/min) | 1693 ± 347 | 1577 ± 330 | 0.178 |
| Resting heart rate (bpm) | 97 ± 15 | 101 ± 15 | 0.287 |
| Peak heart rate (bpm) | 191 ± 6 | 173 ± 11 | <0.001 |
| Maximal chronotropic response index | 0.87 ± 0.06 | 0.69 ± 0.09 | <0.001 |
Note: Comparisons between the 2 groups were performed using the independent-samples test or Mann-Whitney U test.
Abbreviations: bpm = beats per minute; V̇O2peak = peak oxygen uptake; V̇CO2peak = peak carbon dioxide output; W = watt.
Fig. 1.
Heart rate response at rest and during exercise testing in obese individuals with (n = 32) and without (n = 37) CI. Differences in heart rate response were tested using a two-way repeated measures ANOVA. A Bonferroni post hoc comparison test was performed when the between-subjects factor was statistically significant. Data are expressed as mean ± SD. * p < 0.05. ANOVA = analysis of variance; bpm = beats per minute; CI = chronotropic incompetence.
3.3.3. Relation between CRImax and cardiometabolic health in obese adolescents
The CRImax was independently related to blood total cholesterol (standardized coefficient β = –0.332; p = 0.012) and CRP concentration (standardized coefficient β = –0.269; p = 0.039), which together explained about 21% of the variance in CRImax (model r² = 0.201; p = 0.004; adjusted for age, sex, and Tanner stage).
4. Discussion
In the present study, CI was found to be highly prevalent in obese adolescents and related to poor cardiometabolic health as well as exercise intolerance.
CI was prevalent in 32 out of 69 obese adolescents (46% of cohort) and in 3 out of 29 lean adolescents (10% of cohort). These CI prevalence rates were significantly different between lean and obese adolescents (p < 0.01). Moreover, CI starts to appear at a workload that corresponds to about 50% of the V̇O2peak, after which a less steep slope in HR response is present up to peak exercise. These data thus confirm observations in adults with obesity and/or type 2 diabetes mellitus.12,14 This finding is clinically relevant because it shows that in nearly half of the obese adolescents, basing intensities for physical activity and exercise on predicted maximal HR is invalid. In fact, such an approach will lead to an overestimation of the target HR during exercise and physical activity, which should be avoided in order to maintain therapy adherence.37 Based on current findings (Fig. 1), such an overestimation could already be occurring during low-to-moderate-intensity activities.
However, the etiology of CI in obese adolescents remains to be explored in greater detail. In adults with obesity, it has been shown that CI is caused, at least in part, by a suppressed catecholamine synthesis and release during exercise,11 probably due to the exaggerated cortisol response seen in obese individuals38,39 or the exaggerated secretion of (non-)cholinergic and neuropeptides from adipocytes.40 Indeed, previous studies show that the synthesis and release of epinephrine is significantly suppressed in obese adolescents during exercise,41,42 which leads to less stimulation of the atrial sinus node and thus impedes proper HR responses during exercise. Remediation for such an aberrant response in catecholamine release during exercise is currently not available and it is questionable whether this anomaly could be attenuated by exercise training.43 Together with the suppressed catecholamine release, the lower potassium levels observed during exercise in obese individuals11 may also contribute to the manifestation of CI in obese adolescents. However, potassium concentrations were not measured in the present study.
Furthermore, the presence of diastolic dysfunction in obese adolescents has already been shown.19,44,45 Such diastolic dysfunction induces delayed myocardial relaxation, impaired left ventricular filling, and increased myocardial stiffness, all which could result in an inability to increase stroke volume adequately to the degree of effort during exercise, as seen in other populations.14 For these reasons, diastolic dysfunction may be involved in the chronotropic incompetent response seen in this study.
In the present study it was observed that CI was independently related to blood CRP and total cholesterol concentrations (model r² = 0.201, p < 0.05). This is in accordance with the previous research of Huang et al.36 wherein higher concentrations of CRP, as well as N-terminal pro-brain natriuretic peptide, were found in non-obese subjects with CI. The findings of the present study therefore support the hypothesis that obese adolescents with CI may present a pronounced systemic inflammatory status. Together with this higher level of systemic inflammation, cholesterol may play an important role in the development of endothelial damage/dysfunction.46 Steinberg et al.46 showed that even an increase in cholesterol within the (high-) normal range, can contribute to endothelial damage in healthy subjects. This endothelial damage could lead to increased arterial stiffness and, eventually, attenuated HR responses during exercise.47,48 Furthermore, as demonstrated by Lambert et al.,49 an increase in total cholesterol is accompanied by an increase in sympathetic activation, thereby inducing sympathovagal dysbalans, which may lead to the development of CI in young females. Because of the frequent activation of sympathetic nerves, down-regulation of the β-adrenergic receptors may occur, leading to post-synaptic desensitization and thus to a disturbed sympathetic driven HR regulation during exercise.50 However, the exact role of hypercholesterolemia and systemic inflammation in the development or progression of CI in obese adolescents remains to be investigated in detail. Nevertheless, CI clearly relates to a poor cardiometabolic health profile and should be addressed in obese adolescents in order to prevent future cardiovascular events.
CI was also shown to be associated with significant reductions in exercise tolerance, as indicated by a reduction in peak oxygen uptake and peak cycling power output in obese adolescents with CI (p < 0.05). These results confirm previous findings from studies in adult populations.51 It could thus be hypothesized that exercise limitations in obese adolescents may be due to central factors, as they are in adults, and not simply due to skeletal muscle dysfunction.
This study may have been prone to some limitations. First, catecholamine and potassium levels were not measured but should be included in further research. In addition, prospective studies need to confirm measurements regarding autonomic function as well as one of the hypotheses that autonomic imbalance is a key determinant of HR behavior during exercise.
In conclusion, the present study shows that CI is more common in the current cohort of obese adolescents, and relates to systemic inflammation and exercise intolerance.
Acknowledgments
We would like to thank all adolescents for their participation and all parents for their guidance during this study. Furthermore, we thank the clinicians from the department of pediatrics at the Jessa Hospital for their ongoing support.
Acknowledgments
Authors’ contributions
WMAF, CK, NM, KV, and LvR contributed to the acquisition, analysis, or interpretation of data for the work and drafted the manuscript; BOE and PD contributed to the conception or design of the work and critically revised the manuscript; RZ contributed to the acquisition, analysis, or interpretation of data for the work and critically revised the manuscript; GM and DH contributed to the conception or design of the work, contributed to the acquisition, analysis, or interpretation of data for the work, and critically revised the manuscript. All authors have read and approved the final version of the manuscript, and agreed with the order of presentation of the authors.
Competing interests
Prof. D. Hansen discloses receiving personal remuneration for consultancy and/or lectures from Johnson and Johnson outside the scope of this work. All the support had no involvement in the study design and writing of the manuscript or the decision to submit it for publication. All other authors declared that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128. 9 million children, adolescents, and adults. The Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132:1098–1104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulgarón ER. Childhood obesity: A review of increased risk for physical and psychological comorbidities. Clin Ther. 2013;35:A18–A32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruyndonckx L, Hoymans VY, Van Craenenbroeck AH, et al. Assessment of endothelial dysfunction in childhood obesity and clinical use. Oxid Med Cell Longev. 2013;2013:174782. doi: 10.1155/2013/174782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neef M, Weise S, Adler M, et al. Health impact in children and adolescents. Best Pract Res Clin Endocrinol Metab. 2013;27:229–238. doi: 10.1016/j.beem.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: Public-health crisis, common sense cure. The Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 7.Hansen D, Marinus N, Remans M, et al. Exercise tolerance in obese vs. lean adolescents: A systematic review and meta-analysis. Obes Rev. 2014;15:894–904. doi: 10.1111/obr.12202. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson M, Michallet AS, Estève F, et al. Ventilatory responses to exercise training in obese adolescents. Respir Physiol Neurobiol. 2012;184:73–79. doi: 10.1016/j.resp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Nadeau KJ, Zeitler PS, Bauer TA, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen D, Dendale P. Modifiable predictors of chronotropic incompetence in male patients with type 2 diabetes. J Cardiopulm Rehabil Prev. 2014;34:202–207. doi: 10.1097/HCR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 11.Salvadori A, Fanari P, Giacomotti E, et al. Kinetics of catecholamines and potassium, and heart rate during exercise testing in obese subjects. Heart rate regulation in obesity during exercise. Eur J Nutr. 2003;42:181–187. doi: 10.1007/s00394-003-0409-3. [DOI] [PubMed] [Google Scholar]
- 12.Gondoni LA, Titon AM, Nibbio F, Augello G, Caetani G, Liuzzi A. Heart rate behavior during an exercise stress test in obese patients. Nutr Metab Cardiovasc Dis. 2009;19:170–176. doi: 10.1016/j.numecd.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Brubaker PH, Kitzman DW. Chronotropic incompetence: Causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keytsman C, Dendale P, Hansen D. Chronotropic incompetence during exercise in type 2 diabetes: Aetiology, assessment methodology, prognostic impact and therapy. Sports Med. 2015;45:985–995. doi: 10.1007/s40279-015-0328-5. [DOI] [PubMed] [Google Scholar]
- 15.Arbit B, Azarbal B, Hayes SW, et al. Prognostic contribution of exercise capacity, heart rate recovery, chronotropic incompetence, and myocardial perfusion single-photon emission computerized tomography in the prediction of cardiac death and all-cause mortality. Am J Cardiol. 2015;116:1678–1684. doi: 10.1016/j.amjcard.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 17.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 18.Fukuma N, Kato K, Munakata K, et al. Baroreflex mechanisms and response to exercise in patients with heart disease. Clin Physiol Funct Imaging. 2012;32:305–309. doi: 10.1111/j.1475-097X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franssen WMA, Beyens M, Hatawe TA, et al. Cardiac function in adolescents with obesity: Cardiometabolic risk factors and impact on physical fitness. Int J Obes (Lond) 2019;43:1400–1410. doi: 10.1038/s41366-018-0292-x. [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes(Lond) 2006;30:598–602. doi: 10.1038/sj.ijo.0803232. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Green PJ. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 23.Clasey JL, Bradley KD, Bradley JW, Long DE, Griffith JR. A new BIA equation estimating the body composition of young children. Obesity (Silver Spring) 2011;19:1813–1817. doi: 10.1038/oby.2011.158. [DOI] [PubMed] [Google Scholar]
- 24.Houtkooper LB, Lohman TG, Going SB, Howell WH. Why bioelectrical impedance analysis should be used for estimating adiposity. Am J Clin Nutr. 1996;64(Suppl. 3):S436–448. doi: 10.1093/ajcn/64.3.436S. [DOI] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bervoets L, Van Noten C, Van Roosbroeck S, et al. Reliability and validity of the Dutch Physical Activity Questionnaires for Children (PAQ-C) and Adolescents (PAQ-A) Arch Public Health. 2014;72:47. doi: 10.1186/2049-3258-72-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003;88:1019–1023. doi: 10.1210/jc.2002-021127. [DOI] [PubMed] [Google Scholar]
- 29.Vogelzangs N, van der Kallen CJH, van Greevenbroek MMJ, et al. Metabolic profiling of tissue-specific insulin resistance in human obesity: Results from the Diogenes study and the Maastricht Study. Int J Obes (Lond) 2020;44:1376–1386. doi: 10.1038/s41366-020-0565-z. [DOI] [PubMed] [Google Scholar]
- 30.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89:1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 31.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bongers BC, Hulzebos EH, Helbing WA, Ten Harkel ADj, van Brussel M, Takken T. Response profiles of oxygen uptake efficiency during exercise in healthy children. Eur J Prev Cardiol. 2016;23:865–873. doi: 10.1177/2047487315611769. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman K, Stringer WW, Casaburi R, Koike A, Cooper CB. Determination of the anaerobic threshold by gas exchange: Biochemical considerations, methodology and physiological effects. Z Kardiol. 1994;83(Suppl. 3):S1–12. [PubMed] [Google Scholar]
- 34.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 35.Marinus N, Bervoets L, Massa G, et al. Altered gas-exchange at peak exercise in obese adolescents: implications for verification of effort during cardiopulmonary exercise testing. J Sports Med Phys Fitness. 2017;57:1687–1694. doi: 10.23736/S0022-4707.16.06607-X. [DOI] [PubMed] [Google Scholar]
- 36.Huang PH, Leu HB, Chen JW, et al. Comparison of endothelial vasodilator function, inflammatory markers, and N-terminal pro-brain natriuretic peptide in patients with or without chronotropic incompetence to exercise test. Heart. 2006;92:609–614. doi: 10.1136/hrt.2005.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen D, Hens W, Peeters S, et al. Physical therapy as treatment for childhood obesity in primary health care: Clinical recommendation from AXXON (Belgian Physical Therapy Association) Phys Ther. 2016;96:850–864. doi: 10.2522/ptj.20150206. [DOI] [PubMed] [Google Scholar]
- 38.Del Rio G. Adrenomedullary function and its regulation in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl. 2):S89–S91. doi: 10.1038/sj.ijo.0801287. [DOI] [PubMed] [Google Scholar]
- 39.Mannelli M, Lanzillotti R, Pupilli C, Ianni L, Conti A, Serio M. Adrenal medulla secretion in Cushing's syndrome. J Clin Endocrinol Metab. 1994;78:1331–1335. doi: 10.1210/jcem.78.6.8200934. [DOI] [PubMed] [Google Scholar]
- 40.Livett BG, Marley PD. Noncholinergic control of adrenal catecholamine secretion. J Anat. 1993;183:277–289. [PMC free article] [PubMed] [Google Scholar]
- 41.Eliakim A, Nemet D, Zaldivar F, et al. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol (1985) 2006;100:1630–1637. doi: 10.1152/japplphysiol.01072.2005. [DOI] [PubMed] [Google Scholar]
- 42.Zouhal H, Lemoine-Morel S, Mathieu ME, Casazza GA, Jabbour G. Catecholamines and obesity: Effects of exercise and training. Sports Med. 2013;43:591–600. doi: 10.1007/s40279-013-0039-8. [DOI] [PubMed] [Google Scholar]
- 43.Hansen D, Meeusen R, Mullens A, Dendale P. Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity. Sports Med. 2012;42:415–431. doi: 10.2165/11599590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Batalli-Këpuska A, Bajraktari G, Zejnullahu M, et al. Abnormal systolic and diastolic myocardial function in obese asymptomatic adolescents. Int J Cardiol. 2013;168:2347–2351. doi: 10.1016/j.ijcard.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Ingul CB, Tjonna AE, Stolen TO, Stoylen A, Wisloff U. Impaired cardiac function among obese adolescents: Effect of aerobic interval training. Arch Pediatr Adolesc Med. 2010;164:852–859. doi: 10.1001/archpediatrics.2010.158. [DOI] [PubMed] [Google Scholar]
- 46.Steinberg HO, Bayazeed B, Hook G, Johnson A, Cronin J, Baron AD. Endothelial dysfunction is associated with cholesterol levels in the high normal range in humans. Circulation. 1997;96:3287–3293. doi: 10.1161/01.cir.96.10.3287. [DOI] [PubMed] [Google Scholar]
- 47.De Sutter J, Van de Veire N, Elegeert I. Chronotropic incompetence: Are the carotid arteries to blame? Eur Heart J. 2006;27:897–898. doi: 10.1093/eurheartj/ehi712. [DOI] [PubMed] [Google Scholar]
- 48.SY Jae, Fernhall B, Heffernan KS, et al. Chronotropic response to exercise testing is associated with carotid atherosclerosis in healthy middle-aged men. Eur Heart J. 2006;27:954–959. doi: 10.1093/eurheartj/ehi832. [DOI] [PubMed] [Google Scholar]
- 49.Lambert E, Straznicky N, Sari CI, et al. Dyslipidemia is associated with sympathetic nervous activation and impaired endothelial function in young females. Am J Hypertens. 2013;26:250–256. doi: 10.1093/ajh/hps016. [DOI] [PubMed] [Google Scholar]
- 50.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 51.Salvadori A, Fanari P, Mazza P, Agosti R, Longhini E. Work capacity and cardiopulmonary adaptation of the obese subject during exercise testing. Chest. 1992;101:674–679. doi: 10.1378/chest.101.3.674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.