Figure.
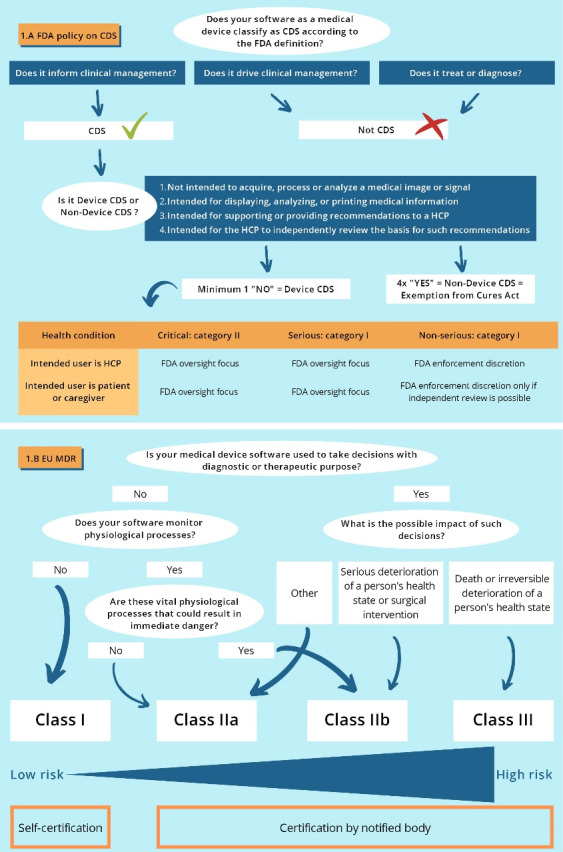
Infographic on Food and Drug Administration Policy on Clinical Decision Support According to the Food and Drug Administration Guidance Specifically for Clinical Decision Support8 (A) and on the Classification of Medical Device Software According to the European Union Medical Device Regulation (B). Abbreviations: CDS, clinical decision support; FDA, Food and Drug Administration; HCP, healthcare professional; MDR, Medical Device Regulation; EU, European Union. “FDA Enforcement Discretion” indicates that the FDA does not intend to enforce compliance, and “FDA oversight focus” indicates that the focus is on regulating CDS software by the FDA. In the United States, CDS functions belong to category I or II depending on the health condition and type of intended user. In the EU, medical device software with the lowest risk is classified as class I device, whereas class III represents the highest risk category. Only class I devices can be self-certified, other devices must be certified by a notified body.
