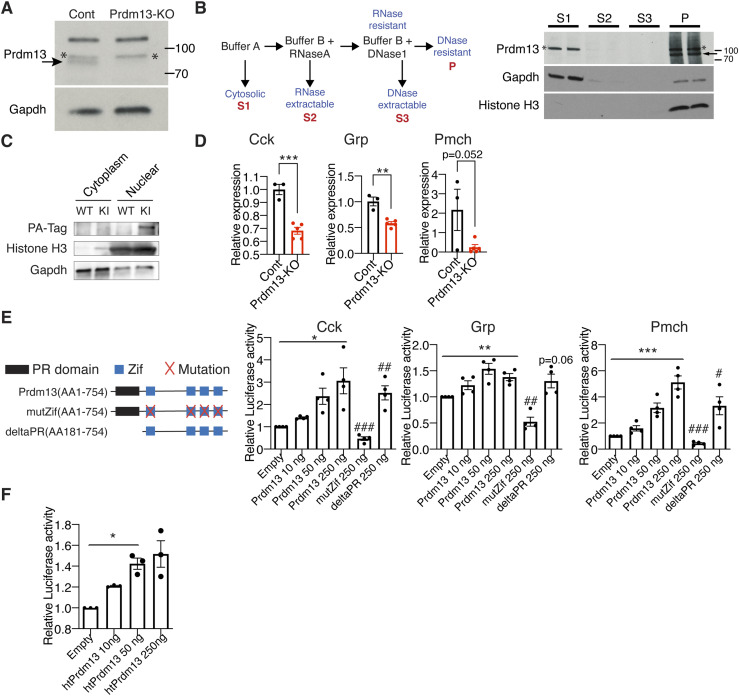
Figure 7. Prdm13 in the DMH is a transcription factor.
(A) Western blot of Prdm13 in DMH collected by laser microdissection from DMH-specific Prdm13-KO and Cont mice (n = 4 mice/lane). The arrow indicates the band for Prdm13; asterisks (*) indicate non-specific bands. (B) Schematic of fractionation protocol from mouse hypothalami (left). Western blot of Prdm13 in hypothalamic fractions of C57BL/6J mice (right). Hypothalami from two C57BL/6J female mice were combined for each lane, and 8% equivalent of each fraction was run on the gel. Cytoplasmic supernatant (S1), RNase-extractable supernatant (S2), DNase-extractable supernatant (S3), and insoluble pellet (P) were run each lane. The arrow indicates the band for Prdm13; asterisks (*) indicate non-specific bands. (C) Western blot of Prdm13 in hypothalamic fractions of Prdm13-PA-Tag (KI) and WT mice. Cytosolic and nuclear fractions were run each lane as indicated. (D) Expression of Cck, Grp, and Pmch mRNA in the DMH of DMH-Prdm13-KO (Prdm13-KO) and control (Cont) mice (n = 3–5). Values are shown as means ± S.E., listed P-value, **P < 0.01 and ***P < 0.001 by unpaired t test. (E) Transcriptional activity of Prdm13-202 and Prdm13-mutants for the luciferase reporter vector containing the promoter region of Cck, Grp, and Pmch. Schematic representation of Prdm13-202 and Prdm13-mutants are shown above. NIH3T3 cells were co-transfected with 250 ng of luciferase reporter plasmid and plasmid expressing Prdm13-202 (Prdm13), Prdm13-Zif mutant (mutZif), or Prdm13-deltaPR mutant (deltaPR). Obtained luminescence was normalized to total protein concentration (n = 3, four individual experiments). Values are shown as means ± S.E., listed P-value, *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Bonferroni’s post hoc test, #P < 0.05 and ##P < 0.01 and ###P < 0.001 by unpaired t test. (F) Transcriptional activity of hypothalamic Prdm13 (htPrdm13) for the luciferase reporter plasmid containing the promoter region of Cck. NIH3T3 cells were co-transfected with 250 ng of reporter plasmid and 10, 50, or 250 ng of htPrdm13-expressing plasmid. Obtained luminescence was normalized to total protein concentrations (n = 3, three individual experiments). Values are shown as means ± S.E., *P < 0.05 by one-way ANOVA with Bonferroni’s post hoc test.