Figure 1. Comparison of participant identification of MRD status between Stages 1 and 2.
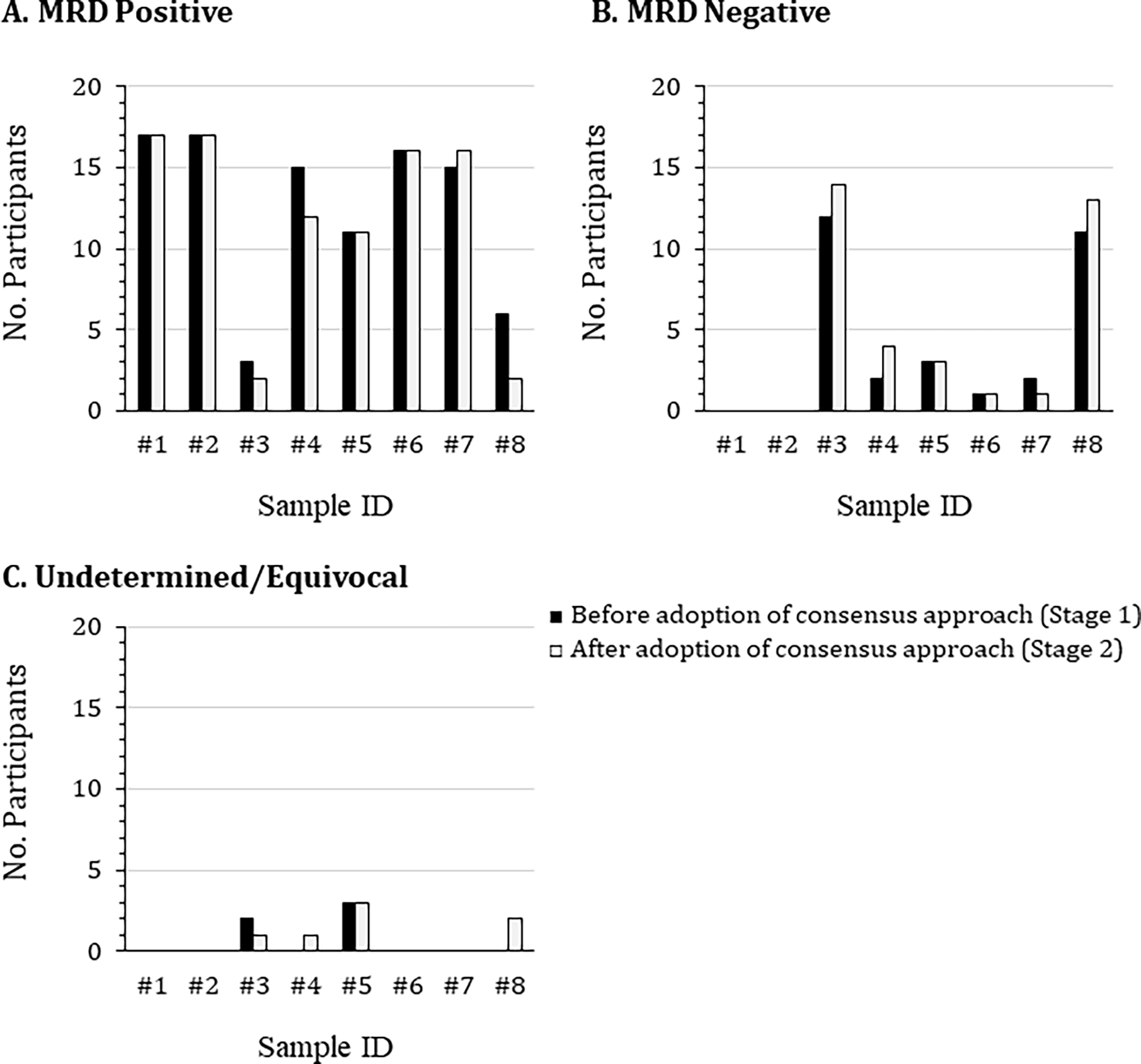
Eight de-identified flow cytometric standard (FCS) data files were distributed to 17 participants at the beginning of the study. These files related to bone marrow aspirate samples collected at Roswell Park from patients with suspected MM MRD or with no known hematological malignancy. The samples were stained using a 2 tube 8-color panel containing consensus markers agreed by a joint initiative of the International Clinical Cytometry Society and European Society for Clinical Cell Analysis. Participants first analyzed the FCS files using their own laboratory-established protocol (black bars), then repeated the analysis using the agreed upon draft consensus analysis protocol (white bars), concluding whether the samples were either (A) MRD positive, (B) MRD negative, or (C) undetermined/equivocal.
