Abstract
Purpose
Individuals with TBI are at risk of intracranial hypertension (ICH), and monitoring of intracranial pressure (ICP) is usually indicated. However, despite many new noninvasive devices, none is sufficiently accurate and effective for application in clinical practice, particularly in the management of TBIs. This study aimed to compare the noninvasive Brain4Care system (nICP) with invasive ICP (iICP) curve parameters in their ability to predict ICH and functional prognosis in severe TBI.
Methods
Observational, descriptive-analytical, and prospective study of 22 patients between 2018 and 2021, simultaneously monitored with nICP and iICP. The independent variables evaluated were the presence of ICH and functional prognoses. The dependent variables were the P2/P1 pressure ratio metrics, time to peak (TTP), and TTP × P2/P1.
Results
We found a good nonlinear correlation between iICP and nICP waveforms, despite a moderate Pearson’s linear correlation. The noninvasive parameters of P2/P1, P2/P1 × TTP, and TTP were not associated with outcomes or ICH. The nICP P2/P1 ratio showed sensitivity/specificity/accuracy (%) of 100/0/56.3, respectively for 1-month outcomes and 77.8/22.2/50 for 6-month outcomes. The nICP TTP ratio had values of 100/0/56.3 for 1-month and 99.9/42.9/72.2 for 6-month outcomes. The nICP P2/P1 × TTP values were 100/0/56.3 for 1-month outcomes and 81.8/28.6/61.1 for 6-month outcomes.
Conclusion
Brain4Care’s noninvasive method showed low specificity and accuracy and cannot be used as the sole means of monitoring ICP in patients with severe TBI. Future studies with a larger sample of patients with P2 > P1 and new nICP curve parameters are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00701-023-05580-z.
Keywords: Traumatic brain injury, Intracranial pressure, Non-invasive techniques, Intracranial hypertension, Prognosis
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, affecting mainly individuals of working age. It is a complex health condition with considerable economic and social costs and, therefore a public health problem, especially in developing countries [13]. The monitoring of intracranial pressure (ICP) is a cornerstone of the clinical management of severe TBI. The maintenance of intracranial pressure below 22 mmHg is vital to TBI interventions such as surgeries or the modification of hemodynamic parameters [12, 15].
The gold standard of ICP monitoring in TBI is the surgical insertion of an intracranial sensor to obtain measurements [39]. The three main sites for sensor placement are intraventricular, intraparenchymal, and subdural [39]. Although useful for monitoring patients with severe head trauma, invasive ICP monitoring is associated with a number of complications, including hemorrhage, obstruction, mispositioning, infection, and, in asymmetric hemispheric lesions, a loss of accuracy. It also necessitates a neurosurgical procedure [4].
Absolute ICP values are clinically useful and are applied in several protocols. However, the diagnostic significance of other parameters of the ICP wave is often underestimated. The ICP waveform morphology has its own tracing, similar to that of the arterial pulse waveform, with three frequent peaks: the percussion wave (P1), the tidal wave (P2), and the dicrotic wave (P3) [3]. Another useful value is the time to peak (TTP), which is defined as the time between the onset of the ICP wave and its highest value [9].
The first mention in the literature of these three components of the ICP curve (P1, P2, and P3 peaks) was by Gega et al. [30] Miller et al. went on to demonstrate that an increase in the P2 component that exceeded P1 corresponded to an increase in the ICP wave [38]. Contant et al. were the first to consider that the rounded shape of the ICP, with centering of the wave peak (calculated by them with the spectral centroid but calculated in the present work using TTP), could be related to intracranial hypertension [16] (Fig. 1).
Fig. 1.

Behavior of P1, P2, and P3 waves in a compliant and non-compliant brain, in the lower curve the wave P1 > P2 (normal brain) and smaller TTP and in the upper curve P2 > P1 (lack of compliance of the system) and higher TTP
However, despite new devices appearing in the medical literature each year that promise to be noninvasive alternatives for the measurement of ICP, none, as yet, is sufficiently accurate and effective for widespread use in clinical practice, particularly in the management of TBI [48]. Currently, most noninvasive devices for monitoring ICP in TBI use the following measures: pressure on the anterior fontanelle, diameter of the optic nerve sheath, venous ophthalmodynamometry, tympanometry, tissue resonance analysis, tonometry, acoustoelasticity, otoacoustic emissions, transcranial Doppler, electroencephalogram (EEG), near-infrared spectroscopy, and pupillometry. None of these approaches is widely used in clinical practice [34, 36, 47].
Since 2013, many articles have been published on the use of the Brain4Care® system in various diseases with variable results [2, 5, 8, 10, 19, 27, 40–44]. The system uses a strain gauge sensor on the scalp, enabling noninvasive monitoring of ICP (nICP). However, to the best of our knowledge, the present study is the first prospective comparison of this system with standard invasive devices (iICP) in TBI.
This work aimed to compare the accuracy of this nICP (Brain4Care) device with that of invasive ICP (iICP) curve parameters (the gold standard) in the prediction of intracranial hypertension (ICH) and functional prognoses in patients with severe TBI. Based on this comparison, we aimed to determine the potential applicability of nICP monitoring in clinical practice.
Material and methods
Patients
This was an observational, descriptive-analytical, and prospective study conducted in a tertiary hospital that receives TBI referrals. Patients were simultaneously monitored with iICP and nICP, and the waveforms and clinical parameters of the two were compared.
The study was conducted between March 2018 and May 2021. Inclusion criteria were age over 14 years, diagnosis of severe TBI, and clinical indication for ICP monitoring (with parenchymal and ventricular catheters). Exclusion criteria were decompressive craniectomy or a surgical approach to cerebral contusions prior to sensor placement for iICP monitoring, skin conditions, or other clinical conditions that precluded the positioning of sensors for nICP monitoring, complex or misaligned skull fractures that caused instability and deformation. There were 22 patients included in the study. All patients were followed up on an outpatient basis for up to 6 months.
The independent variables evaluated were the presence of ICH (defined as ICP greater than 22 mmHg for more than 5 min [12]) and functional prognoses, as determined by Glasgow Outcome Scale (GOS) scores (scoring 1–5, where 1, death and 5, good recovery, minor neurological deficits) at 1, 3, and 6 months, obtained from patient medical records or by contacting the family by telephone. The dependent variables were the P2/P1 ratio metrics, TTP, and TTP × P2/P1.
Ethics statement and patient consent
This study was conducted in accordance with the tenets of the 2013 revision of the Declaration of Helsinki and was approved by the Ethics Committee of the Clinical Hospital of the Faculty of Medicine of Ribeirão Preto (3.994.848). Written informed consent to participation and publication was obtained from the legal representative of each patient.
Monitoring
All patients in this study underwent simultaneous invasive (gold standard) and noninvasive ICP monitoring. For iICP monitoring, a sensor (invasive Codman®-Integra, Raynham, MA, USA) was inserted through Kocher’s point in the affected cerebral hemisphere and, in cases of diffuse edema, in the right cerebral hemisphere. Invasive monitoring was continued for as long as clinically justified.
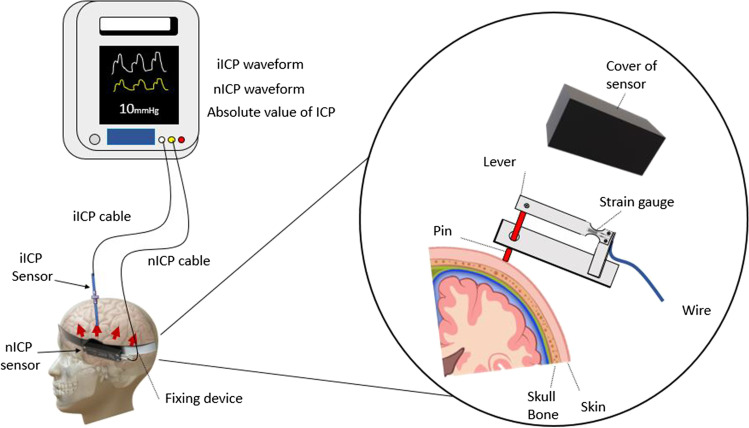
For nICP monitoring, the Brain4Care® (São Carlos, Sao Paulo, Brazil) system was used. When using this system, an electrical resistance strain gauge is applied to the external surface of the skull. This is based on the hypothesis that, as the skull is not completely rigid, changes in ICP result in small but measurable expansions of the skull, with no need for a surgical implant [11]. A sensor is placed on the skin and attached with elastic tape. This is able to measure cranial deformation through a system of built-in strain gauges, generating a noninvasive ICP curve (Fig. 2).
Fig. 2.
Infographic illustrating invasive and noninvasive monitoring. A brace is installed circumferentially on the skull. The sensor transmits invasive and non-invasive information obtained to a monitor simultaneously, then the intensivist can use the invasive values for clinical performance and the non-invasive data are stored for research. ICP, intracranial pressure; TTP, time to peak
The noninvasive system (sensor and equipment) was applied by the researchers at the bedside in the intensive care unit after the procedure for invasive monitoring had been performed. The noninvasive monitor can record ICP information (number and waveform) obtained from both invasive and noninvasive methods, providing information for immediate interpretation and later analysis.
Noninvasive monitoring was performed for as long as iICP measurement was necessary. Rotation of the area of installation of the noninvasive device was carried out every 2 h by the nursing team under the supervision of the responsible researcher and collaborators to avoid pressure-induced skin injury. No wave difference was found in different measurement sites.
Initially, all data obtained (total of 12,081 min of monitoring) from the nICP and iICP curves were automatically processed using Brain4Care Analytics software [6] into individual units, each representing a wave of nICP and iICP with P1, P2, and TTP. These units were first grouped into 1-min intervals and then into 10-min intervals and the averages of P2/P1 and TTP were calculated, generating 3565 average pulses. Afterward, a noise signal filter was applied to remove artifacts, leaving 1241 average pulses. These were processed to obtain receiver operating characteristic (ROC) curves. ROC curve analysis was performed using software developed by Johns Hopkins University [25].
The calculations were performed using category 1 analysis, which accounts for the severity of each parameter, using a scoring scale of 1–6, with known negatives scoring 1 and certain positives as 6. Table 1 shows the graduations and values used for the parameters analyzed.
Table 1.
Parameters used to create the ROC curve according to Johns Hopkins University School of Medicine Online ROC Curve Calculator (http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html)
| Note | Description | P2/P1 ratio | TTP | P2/P1 × TTP | GOS |
|---|---|---|---|---|---|
| 1 | Definitely negative | < 1 | < 0.23 | < 0.31 | 5 |
| 2 | Probably negative | 1 | 0.23 | 0.31 | - |
| 3 | Possibly negative | 1.1 | 0.24 | 0.32 | - |
| 4 | Possibly positive | 1.2 | 0.25 | 0.33 | 4 |
| 5 | Probably positive | 1.3 | 0.26 | 0.34 | 3 |
| 6 | Definitely positive | ≥1.4 | ≥0.27 | ≥ 0.35 | < 3 |
TTP, time to peak; GOS, glasgow outcome scale
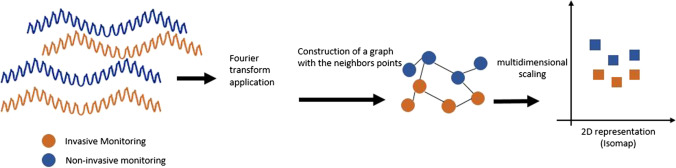
We applied Isomap nonlinear dimension reduction to all points obtained from the nICP curve, reducing them to a low-dimensional set projected onto a graph. This technique projects the pulses into a bi-dimensional space (K1 and K2) to compare the entire waveform shapes of the invasive and noninvasive ICP signals [46] (Fig. 3).
Fig. 3.
Flowchart showing the steps used to calculate and design Isomap. Fourier transform is a mathematical model to transform the signals from time domain to frequency domain
Isomap is a non-linear dimensionality reduction method. It is a widely used low-dimensional embedding method. Isomap is used to calculate a low-dimensional quasi-isometric embedding obtained from a set of high-dimensional data points (ICP P1, P2, and P3 points and the P2/P1 and TTPA). In the present study, all the points obtained from the nICP curve in its wave-by-wave record is a high-dimensional set that can be reduced to a low-dimensional set and projected on a graph. The Isomap algorithm provides a method for estimating the intrinsic geometry of a dataset based on a rough estimate of each point’s neighbors. Isomap is highly efficient and generally applicable to a wide range of data sources and dimensionality especially meteorology and astrophysics which deal with high dimensional datasets [46].
Results
Twenty-five patients were monitored between March 2018 and March 2021, but only 22 were included in the study (Appendix) as the data from three patients (#1, #6, and #9) were unusable due to poor quality monitoring signals. Intraventricular iICPs were kept closed during the study.
Linear and nonlinear ICP comparisons
The values of all validated pulses of all patients were obtained both invasively and noninvasively so, after filtering by the signal-to-noise ratio, the nonlinear Isomap technique was used to project the pulses into a two-dimensional space. In this way, it was possible to evaluate the behavior of the iICP and nICP waves in relation to K1 and K2 (projections in two Isomap planes), P2/P1, TTP, and P2/P1 × TTP. The Pearson’s linear correlations (ρ) for K2 and P2/P1 showed moderate correction, with values of 0.49 and 0.52, respectively. Nonlinear analysis of the MI found a relationship between the curves with respect to K2 (MI = 0.65), TTP (MI = 0.69), and P2/P1 × TTP (MI = 0.75) (Table 2).
Table 2.
Pearson’s correlation between iICP and nICP in relation to K1 and K2 (Isomap), TTP, P2/P1, and P2/P1 × TTP
| K1 | K2 | TTP | P2/P1 | P2/P1 × TTP | |
|---|---|---|---|---|---|
| ρ | 0.312 | 0.491 | − 0.013 | 0.525 | 0.173 |
| MI | 0.444 | 0.653 | 0.694 | 0.366 | 0.751 |
ρ ranges from − 1 to + 1, with zero unrelated between the variables and the extremes as perfect correlations
MI, mutual information normalized boo from 0 to 1 with 1 being a perfect correlation
TTP, time to peak; iICP, invasive intracranial pressure; nICP, noninvasive intracranial pressure
Highlighted are the correlations considered strong
In bold correlations considered strong
ICP waveforms and absolute iICP values
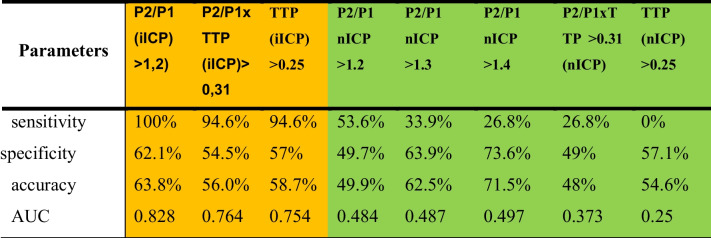
According to the ROC curve, the iICP waveform in the context of ICH (defined as > 22 mmHg) had a sensitivity of 100%, specificity of 62.1%, and accuracy of 63.8% to predict ICP above this value. The invasive P2/P1 × TTP ratio was statistically significant for the prediction of ICP > 22 mmHg when its value was greater than 0.31, with a sensitivity of 96.4%, specificity of 54.5%, and accuracy of 56%. However, the nICP parameters showed no strong relationships between P2/P1 > 1.2, P2/P1 > 1.3, P2/P1 > 1.4, P2/P1 × TTP > 0.31, and TTP > 0.25 (Table 3).
Table 3.
Relation obtained by the ROC curve between iICP/nICP parameters and ICP greater than 22 mmHg. TTP, time to peak; iICP, invasive intracranial pressure; nICP noninvasive intracranial pressure. Yellow: invasive ICP. Green: non-invasive ICP
ICP waveforms and prognosis
A comparison of the results with the clinical outcomes measured by the GOS found that the worsening of the mean value of iICP was associated with outcomes (Table 4). This value showed a sensitivity of 100% for outcomes at 1 month and 88.9% for outcomes at 6 months (cut-off value of iICP of ≥ 22 mmHg).
Table 4.
Relationship between mean ICP and functional outcome at 1, 3, and 6 months
| iICP-average value | |||
|---|---|---|---|
| GOS 1m | GOS 6m | ||
| Average value | Accuracy | 43.80% | 61.10% |
| Sensitivity | 100.00% | 88.90% | |
| Specificity | 0.00% | 33.30% | |
| AUC | 0.75 | 0.75 | |
In bold correlations considered strong
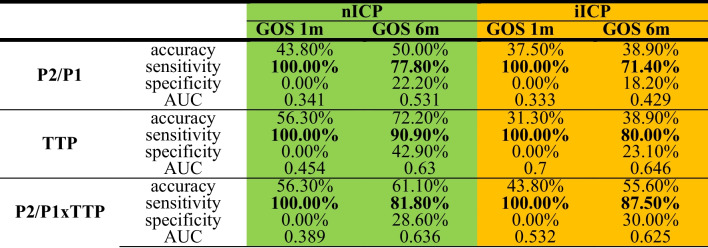
Regarding the ICP waveform parameters, the nICP P2/P1 ratio showed a sensitivity of 100% for outcomes at 1 month and 77.8% for outcomes at 6 months, while that of the iICP showed sensitivities of 100% and 71.4% for the same outcomes. The nICP TTP ratio had a sensitivity of 100% for outcomes at 1 month and 90.9% for outcomes at 6 months, while the TTP ratio of the iICP had sensitivities of 100% and 80.0% for the same outcomes. The nICP P2/P1 × TTP had a sensitivity of 100% for outcomes at 1 month and 81.8% for outcomes at 6 months, while the iICP P2/P1 × TTP had sensitivities of 100% and 87.5% for the same outcomes (Table 5).
Table 5.
Relationship between the nICP and iICP curves with and their parameters P2/P2, TTP, and P2/P1 × TTP. iICP, invasive intracranial pressure; nICP, noninvasive intracranial pressure; GOS, glasgow outcome scale; TTP, time to peak. Highlighted are the values above 75%. Yellow: invasive ICP. Green: non-invasive ICP
Discussion
An ideal noninvasive system for monitoring ICP is the holy grail of neurocritical care practice and neurosurgery. If available, it would have a wide range of applications in neurology, neuroscience, and translational medicine, from exercise physiology to aerospace medicine [20].
The Brain4Care noninvasive monitoring device was cited in 50 papers between 2013 and 2022. Apart from experimental and non-original papers, most of these articles were piloting the use of the device in patients with various neurological and non-neurological conditions. These included cryptococcal meningitis [5], renovascular hypertension [26], pediatric hydrocephalus [2], obesity [8], critical COVID-19 [10], end-stage renal disease [41], pregnancy [19], patients sedated for gastrointestinal endoscopy [24], sedentary chronic stroke patients [40], hemodialysis patients [42], prostatectomy surgery patients [45], fibromyalgia [33], cardiovascular surgery patients [1], diabetes and chronic kidney disease [35], and migraine [14]. To the best of our knowledge, this is the first prospective study to compare the Brain4Care noninvasive method with the invasive gold standard in TBI.
As has been previously noted, ICP is much more than a number. Its complex waveform has led some previous authors to compare invasive and noninvasive ICP curves. Frigieri et al. used Isomap analysis to analyze the data of seven patients monitored by iICP and nICP (Brain4Care) simultaneously with invasive radial arterial pressure. The patients had spontaneous hemorrhages, TBIs, or brain tumors. A strong correlation was found between iICP and nICP, and there was a statistically significant difference when these waveforms were compared to the arterial invasive blood pressure wave [27].
Gomes et al. compared the iICP and nICP curves and showed that the correlation between the two is probably nonlinear in nature [31]. Di Leva et al. demonstrated that nonlinear analysis can also be a reliable means of describing ICP signals over time [23].
Moraes et al. used the Brain4Care sensor simultaneously with iICP in stroke patients (subarachnoid hemorrhage, intracerebral hemorrhage, or ischemic stroke) for 30 min and found correlations and agreement between the invasive and noninvasive methods for wave morphology, which were strong for the P2/P1 ratio and moderate for TTP using categoric (κ agreement 88.1% and 71.3%, respectively) and continuous (intraclass correlation coefficient 0.831 and 0.584, respectively) measures. There was a moderate but significant correlation between the mean ICP values obtained from the two techniques (P2/P1 ratio r = 0.427; TTP r = 0.353; p < 0.001 for all). The areas under the curve for estimation of ICH were 0.786 (95% confidence interval [CI], 0.72–0.93) for the P2/P1 ratio and 0.694 (CI95%, 0.60–0.74) for TTP. ICH was observed in 30% of the data monitored [21].
In the present study, linear analysis verified that the nICP and iICP curves behave differently, while in nonlinear analyses such as MI, they are similar. In general, the curves were found to be related in terms of their shape, when considering the variables K2, P2/P1, and P2/P1 × TTP.
In 2018, Frigieri et al. were the first to compare Brain4Care nICP and iICP in a heterogeneous sample of patients and found similarities between the noninvasive and invasive waveforms [27]. To the best of our knowledge, this is the only study besides the present work to compare iICP with Brain4Care nICP in TBI patients.
An interesting approach to the prediction of ICH uses past changes in waveform morphology. Recognizing that most clinical decision-making accounts for only the absolute value of ICP, Hu et al. proposed a technique for the automatic extraction of useful information from the iICP waveform. This is achieved through the detection of the P1, P2, and P3 peaks within the ICP waveform. They named this morphological clustering and analysis of intracranial pressure (MOCAIP) [32].
Using the MOCAIP technique in a sample that included 23 TBI inpatients, ICP pulse morphological metrics were shown to correlate with low cerebral blood flow (CBF), as measured by an intravenous xenon-133 clearance technique. Of particular interest was the association found between high P3 peaks and low FSC. However, the correlation between pulse morphological metrics and low CBF was lower in patients with TBI than those with other diagnoses, such as subarachnoid hemorrhage and hydrocephalus [32]. Unfortunately, in the present study, it was not possible to measure P3 peaks.
A study of TBI patients described the characteristics of iICP plateau waves (sudden and relevant increases in ICP of 40–100 mmHg with durations of 5–20 min). The study observed plateau waves in 44% of patients. It was also observed that abrupt increases in ICP were associated with increases in ICP pulse wave amplitudes and significant decreases in CBF and cerebral oxygenation, despite cardiovascular variables such as blood pressure and heart rate remaining stable. Analysis of the ICP pulse waveform during plateau waves using invasive monitoring found significant increases in amplitude and changes in the shape of ICP pulse components with P1 < P2 > P3 [22].
In the present investigation, the iICP P2/P1 ratio had a sensitivity of 100%, specificity of 62.1%, and accuracy of 63.8% for the prediction of ICP > 22 mmHg. The iICP P2/P1 × TTP ratio showed a significant ability to predict ICP > 22 mmHg, when its value was > 0.31, with a sensitivity of 96.4%, specificity of 54.5%, and accuracy of 56%. The corresponding results obtained using nICP monitoring were less satisfactory.
Therefore, in our sample, the value increases to those deemed indicative of ICH (> 22 mmHg) were not reliably predicted by noninvasive monitoring. Therefore, we must conclude that, although nICP monitoring showed a satisfactory nonlinear relationship with the ICP curve, which is useful data for the mathematical and physical study of ICP monitoring, it did not obtain sufficient statistical strength to predict ICP values indicative of ICH. However, this deficiency in nICP monitoring could be considered a flaw in ICH criteria since the use of a specific ICP cutoff value for ICH does not consider the context of each TBI and the multiple factors that affect brain dynamics and homeostasis. The iICP value of 22 mmHg used in the latest protocols does not allow for variation between individuals, which is likely to depend on multiple intrinsic factors, such as age, sex, and comorbidities, and extrinsic factors, such as systemic trauma severity, type of brain injury, and brain autoregulation capacity [18, 37].
In the present study, the values obtained for P2/P1, TTP, and P2/P1 × TTP may be related to brain compliance. This is the ability of the intracranial space to accommodate changes in volume. The degree of brain compliance affects the relationship between changes in volume and corresponding changes in pressure. Thus, if our values relate to brain compliance, this, in turn, will relate to the temporal evolution of ICP and the prognosis of the TBI patient. Using the Brain4Care method, research has shown that brain compliance decreases as the P2/P1 ratio increases in animal models of spinal subarachnoid infusion [28]. Recent Brain4Care studies report preliminary experience with a black box algorithm that derives a composite index of ICC (intracranial compliance scale—ICS) from the nICP waveform. The primary aim was to evaluate the ICS ability to detect elevations in ICP and the secondary to correlate ICS with early in-hospital mortality (7 days). The authors report that preliminary results suggest that the ICS is a reliable tool for the detection of ICC alterations [7, 29].
When the parameters of the P2/P1, TTP, and P2/P1 × TTP curves were compared with the functional outcomes of our sample, we found that the P2/P1, TTP, and P2/P1 × TTP ratios of both the nICP and iICP showed high sensitivity to the 1 and 6-month outcomes. All nICP specificity values were low for these parameters.
Few studies have tried to establish the relationships between ICP curve parameters and patient prognosis. A study of 56 TBI patients on mechanical ventilation showed that RAP coefficients (which is the compensatory reserve index derived from the mobile correlation between the ICP pulse amplitude and the ICP value itself) close to zero correlate with worse functional prognoses, as measured by GOS, 12 months after the TBI [17]. However, the calculation of this parameter is complex, and it has not been incorporated into clinical practice.
Limitations
A particular limitation of the present study was obtaining participants during the COVID-19 pandemic, which restricted access to intensive care and the resources necessary for invasive ICP monitoring. This also affected the follow-up durations. In addition, automatic extraction of the nICP curve provided fewer parameters for study, such as P1, P2, and their relationships, and presents difficulties with timely interpretation in clinical practice, which can hamper feasibility and lead to subjective interpretations and potential inappropriate conduct, if used indiscriminately.
Other studies of the same noninvasive monitoring device have used the device for shorter periods, while we continuously monitored participants for several days. This increased the incidence of issues with poor device positioning, movement artifacts, and unusable results. Another limitation was the relatively low number of ICH peaks, which limited the analysis of this variable. Also, failure to obtain data on other parameters of neuro and hemodynamic monitoring made the assessment of brain compliance unfeasible. Finally, an important limitation in the use of this noninvasive monitoring device is that it does not allow CSF drainage in the treatment of ICH.
Conclusions
Brain4Care’s noninvasive system cannot be used alone to monitor intracranial pressure in individuals with severe TBI. It is limited by low sensitivity to change prediction in ICH and low specificity in functional evolution prediction. However, further studies that include more individuals with high ICP, other nICP curve parameters, and the assessment of brain compliance could establish reliable cutoff values for the P2/P1, TTP, and P2/P1 × TTP ratios, among other possible parameters. This would then allow greater use of the device in clinical decision-making and prognosis definition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was completed in partial fulfillment of the requirements for a PhD in Science by Dr. Matheus Ballestero.
The authors thank Dr. Gustavo Frigieri and Brain4Care for the provision of the software and hardware used in this study; Dr. Sergio Brasil for brain4care device consulting and data analysis. Dr. Lissa Kido and Bruno Padua for data processing and statistical analysis.
Author contribution
MB: conceptualization; data curation; investigation; methodology; supervision; validation; visualization; roles/writing—original draft; writing—review & editing
CD: data curation; roles/writing—original draft; writing—review & editing
ICNG: data curation; roles/writing—original draft; writing—review & editing
LSG: investigation, data curation
RAMC: investigation, data curation
ELZJ: investigation, data curation
RSO: conceptualization; data curation; investigation; methodology; project administration; supervision; validation; visualization; writing—review & editing
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ayres C, Vellosa JCR. Noninvasive monitoring of intracranial pressure after cardiac surgery: case report. Research, Society and Development. 2022;11(6):e56011629492–e56011629492. doi: 10.33448/rsd-v11i6.29492. [DOI] [Google Scholar]
- 2.Ballestero MFM, Frigieri G, Cabella BCT, de Oliveira SM, de Oliveira RS. Prediction of intracranial hypertension through noninvasive intracranial pressure waveform analysis in pediatric hydrocephalus. Childs Nerv Syst. 2017;33(9):1517–1524. doi: 10.1007/s00381-017-3475-1. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont A. Physiology of the cerebrospinal fluid and intracranial pressure. In: Winn HR, Youmans JR, editors. Youmans and Winn neurological surgery. 7. Philadelphia: Elsevier/Saunders; 2017. pp. 417–423. [Google Scholar]
- 4.Bekar A, Doğan S, Abaş F, Caner B, Korfali G, Kocaeli H, Yilmazlar S, Korfali E. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J Clin Neurosci. 2009;16(2):236–240. doi: 10.1016/j.jocn.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Bollela VR, Frigieri G, Vilar FC, et al. Noninvasive intracranial pressure monitoring for HIV-associated cryptococcal meningitis. Braz J Med Biol Res. 2017;50(9):e6392. doi: 10.1590/1414-431x20176392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brain4Care Brain4care Analytics. https://braincareanalytics.com/. Accessed 9 Aug 2022
- 7.Brasil S, Frigieri G, Taccone FS, Robba C, Solla DJF, de Carvalho Nogueira R, Yoshikawa MH, Teixeira MJ, Malbouisson LMS, Paiva WS (2022) Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J Clin Monit Comput 1–8 [DOI] [PMC free article] [PubMed]
- 8.Brasil S, Renck AC, Taccone FS, et al. Obesity and its implications on cerebral circulation and intracranial compliance in severe COVID-19. Obes Sci Pract. 2021 doi: 10.1002/osp4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil S, Solla DJF, de Nogueira RC, Jacobsen Teixeira M, Malbouisson LMS, Paiva WS. Intracranial compliance assessed by intracranial pressure pulse waveform. Brain Sci. 2021 doi: 10.3390/brainsci11080971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasil S, Taccone FS, Wayhs SY, et al. Cerebral hemodynamics and intracranial compliance impairment in critically ill COVID-19 patients: a pilot study. Brain Sci. 2021 doi: 10.3390/brainsci11070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabella B, Vilela GHF, Mascarenhas S, et al. Validation of a new noninvasive Intracranial pressure monitoring method by direct comparison with an invasive technique. Acta Neurochir Suppl. 2016;122:93–96. doi: 10.1007/978-3-319-22533-3_18. [DOI] [PubMed] [Google Scholar]
- 12.Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 13.Carteri RBK, da Silva RA. Traumatic brain injury hospital incidence in Brazil: an analysis of the past 10 years. Rev Bras Ter Intensiva. 2021;33(2):282–289. doi: 10.5935/0103-507X.20210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherain LGG, de Sena Barbosa MG, de Souza RLV, de Souza Paula R, Campos GR, Silva I, Peixoto E, Rabelo NN (2021) Use of non-invasive sensor to assess intracranial compliance in the management of migraine patients: case report. Mapping Intimacies
- 15.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contant CF, Robertson CS, Crouch J, Gopinath SP, Narayan RK, Grossman RG. Intracranial pressure waveform indices in transient and refractory intracranial hypertension. J Neurosci Methods. 1995;57(1):15–25. doi: 10.1016/0165-0270(94)00106-Q. [DOI] [PubMed] [Google Scholar]
- 17.Czosnyka M, Guazzo E, Whitehouse M, Smielewski P, Czosnyka Z, Kirkpatrick P, Piechnik S, Pickard JD (1996) Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir (Wien) 138(5):531–41; discussion 541 [DOI] [PubMed]
- 18.Czosnyka M, Smielewski P, Timofeev I, Lavinio A, Guazzo E, Hutchinson P, Pickard JD. Intracranial pressure: more than a number. Neurosurg Focus. 2007;22(5):E10. doi: 10.3171/foc.2007.22.5.11. [DOI] [PubMed] [Google Scholar]
- 19.da Silveira D, Rabelo NN, de Sena Barbosa MG, Frigeri G, Vellosa JCR. Intracranial pressure and laboratory parameters in high- and low-risk pregnant women. Surg Neurol Int. 2021;12:250. doi: 10.25259/SNI_109_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Moraes FM, Silva GS. Noninvasive intracranial pressure monitoring methods: a critical review. Arq Neuropsiquiatr. 2021;79(5):437–446. doi: 10.1590/0004-282x-anp-2020-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Moraes FM, Rocha E, Barros FCD, Freitas FGR, Miranda M, Valiente RA, de Andrade JBC, Neto FEAC, Silva GS. Waveform morphology as a surrogate for ICP monitoring: a comparison between an invasive and a noninvasive method. Neurocrit Care. 2022 doi: 10.1007/s12028-022-01477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias C, Maia I, Cerejo A, Varsos G, Smielewski P, Paiva J-A, Czosnyka M. Pressures, flow, and brain oxygenation during plateau waves of intracranial pressure. Neurocrit Care. 2014;21(1):124–132. doi: 10.1007/s12028-013-9918-y. [DOI] [PubMed] [Google Scholar]
- 23.Di Ieva A, Schmitz EM, Cusimano MD. Analysis of intracranial pressure: past, present, and future. Neuroscientist. 2013;19(6):592–603. doi: 10.1177/1073858412474845. [DOI] [PubMed] [Google Scholar]
- 24.Drewnowsk B, Acras RN, Rickli C, Koch MS, Bueno BF, Novak RS, Vellosa JCR. Non-invasive monitoring of intracranial pressure during midazolam-induced moderate sedation for upper gastrointestinal endoscopy. Brazilian J Develop. 2021;7(5):44822–44830. [Google Scholar]
- 25.Eng J, Morgan RH (2017) ROC Analysis. http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html. Accessed 1 Sep 2022
- 26.Fernandes MV, Rosso Melo M, Mowry FE, Lucera GM, Lauar MR, Frigieri G, Biancardi VC, Menani JV, Colombari DSA, Colombari E. Intracranial pressure during the development of renovascular hypertension. Hypertension. 2021;77(4):1311–1322. doi: 10.1161/HYPERTENSIONAHA.120.16217. [DOI] [PubMed] [Google Scholar]
- 27.Frigieri G, Andrade RAP, Dias C, Spavieri DL, Brunelli R, Cardim DA, Wang CC, Verzola RMM, Mascarenhas S. Analysis of a non-invasive intracranial pressure monitoring method in patients with traumatic brain injury. Acta Neurochir Suppl. 2018;126:107–110. doi: 10.1007/978-3-319-65798-1_23. [DOI] [PubMed] [Google Scholar]
- 28.Frigieri G, Andrade RAP, Wang CC, Spavieri D, Lopes L, Brunelli R, Cardim DA, Verzola RMM, Mascarenhas S. Analysis of a minimally invasive intracranial pressure signals during infusion at the subarachnoid spinal space of pigs. Acta Neurochir Suppl. 2018;126:75–77. doi: 10.1007/978-3-319-65798-1_16. [DOI] [PubMed] [Google Scholar]
- 29.Frigieri G, Robba C, Machado FS, Gomes JA, Brasil S. Application of non-invasive ICP waveform analysis in acute brain injury: Intracranial Compliance Scale. ICMx. 2023;11(1):5. doi: 10.1186/s40635-023-00492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gega A, Utsumi S, Iida Y, Iida N, Tsuncda S. Analysis of the wave pattern of CSF pulse wave. In: Shulman K, Marmarou A, Miller JD, Becker DP, Hochwald GM, Brock M, editors. Intracranial Pressure IV. Berlin Heidelberg, Berlin, Heidelberg: Springer; 1980. pp. 188–190. [Google Scholar]
- 31.Gomes I, Shibaki J, Padua B, Silva F, Gonçalves T, Spavieri-Junior DL, Frigieri G, Mascarenhas S, Dias C. Comparison of waveforms between noninvasive and invasive monitoring of intracranial pressure. Acta Neurochir Suppl. 2021;131:135–140. doi: 10.1007/978-3-030-59436-7_28. [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Glenn T, Scalzo F, Bergsneider M, Sarkiss C, Martin N, Vespa P. Intracranial pressure pulse morphological features improved detection of decreased cerebral blood flow. Physiol Meas. 2010;31(5):679–695. doi: 10.1088/0967-3334/31/5/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junior AEA, Carbinatto FM, Tomaz CSR, Bagnato VS. Photosonic treatment and fibromyalgia: the effect on brain compliance-case report. J Nov Physiother. 2022;12(510):2. [Google Scholar]
- 34.Khan MN, Shallwani H, Khan MU, Shamim MS. Noninvasive monitoring intracranial pressure - a review of available modalities. Surg Neurol Int. 2017;8:51. doi: 10.4103/sni.sni_403_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch MS, Drewnowski B, Balzer ER, Baroni G, Schuinski AFM, Vellosa JCR. Alteração da complacência intracraniana em paciente com doença renal crônica e diabetes mellitus T1: um relato de caso. Res, Soc Develop. 2022;11(8):e26811831153–e26811831153. doi: 10.33448/rsd-v11i8.31153. [DOI] [Google Scholar]
- 36.Kristiansson H, Nissborg E, Bartek J, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372–385. doi: 10.1097/ANA.0b013e31829795ce. [DOI] [PubMed] [Google Scholar]
- 37.Lazaridis C, DeSantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD, Czosnyka M. Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg. 2014;120(4):893–900. doi: 10.3171/2014.1.JNS131292. [DOI] [PubMed] [Google Scholar]
- 38.Miller JD, Peeler DF, Pattisapu J, Parent AD. Supratentorial pressures Part II.: Intracerebral pulse waves. Neurol Res. 1987;9(3):198–201. doi: 10.1080/01616412.1987.11739795. [DOI] [PubMed] [Google Scholar]
- 39.Nag DS, Sahu S, Swain A, Kant S. Intracranial pressure monitoring: gold standard and recent innovations. World J Clin Cases. 2019;7(13):1535–1553. doi: 10.12998/wjcc.v7.i13.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai Ocamoto G, Spavieri Junior DL, Matos Ribeiro JA, FrigieriVilela GH, Catai AM, Russo TL. Noninvasive intracranial pressure monitoring in chronic stroke patients with sedentary behavior: a pilot study. Acta Neurochir Suppl. 2021;131:55–58. doi: 10.1007/978-3-030-59436-7_12. [DOI] [PubMed] [Google Scholar]
- 41.Rickli C, Cosmoski LD, Dos Santos FA, Frigieri GH, Rabelo NN, Schuinski AM, Mascarenhas S, Vellosa JCR. Use of non-invasive intracranial pressure pulse waveform to monitor patients with end-stage renal disease (ESRD) PLoS ONE. 2021;16(7):e0240570. doi: 10.1371/journal.pone.0240570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickli C, Koch MS, Bueno BF, Vellosa JCR. Effect of hemodialysis on cerebral compliance assessed in a non-invasive way: case report. Brazilian Journal of Development. 2021;7(1):10169–10177. doi: 10.34117/bjdv7n1-689. [DOI] [Google Scholar]
- 43.Rochetti Bezerra TA, Spavieri Júnior DL, Frigieri G, Brunell R, de Oliveira SM (2018) In-flight analysis of intracranial pressure in pilots undergoing variation in Gz. AAOAJ. 10.15406/aaoaj.2018.02.00042
- 44.Rojas SSO, Ordinola AAM, Veiga VC, de Souza JM. The use of a noninvasive intracranial pressure monitoring method in the intensive care unit to improve neuroprotection in postoperative cardiac surgery patients after extracorporeal circulation. Rev Bras Ter Intensiva. 2021;33(3):469–476. doi: 10.5935/0103-507X.20210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saba GT, Quintão VC, Zeferino SP, Simões CM, Coelho RF, Fazoli A, Nahas W, Vilela GHF, Carmona MJC. Noninvasive intracranial pressure real-time waveform analysis monitor during prostatectomy robotic surgery and Trendelenburg position: case report. Braz J Anesthesiol. 2021;71(6):656–659. doi: 10.1016/j.bjane.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tenenbaum JB, de Silva V, Langford JC. A global geometric framework for nonlinear dimensionality reduction. Science. 2000;290(5500):2319–2323. doi: 10.1126/science.290.5500.2319. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, He W, Chen W-M, Zhu L. Research on simulation and experiment of noninvasive intracranial pressure monitoring based on acoustoelasticity effects. Med Devices (Auckl) 2013;6:123–131. doi: 10.2147/MDER.S47725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J, Webster JG. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas. 2017;38(8):R143–R182. doi: 10.1088/1361-6579/aa7256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.