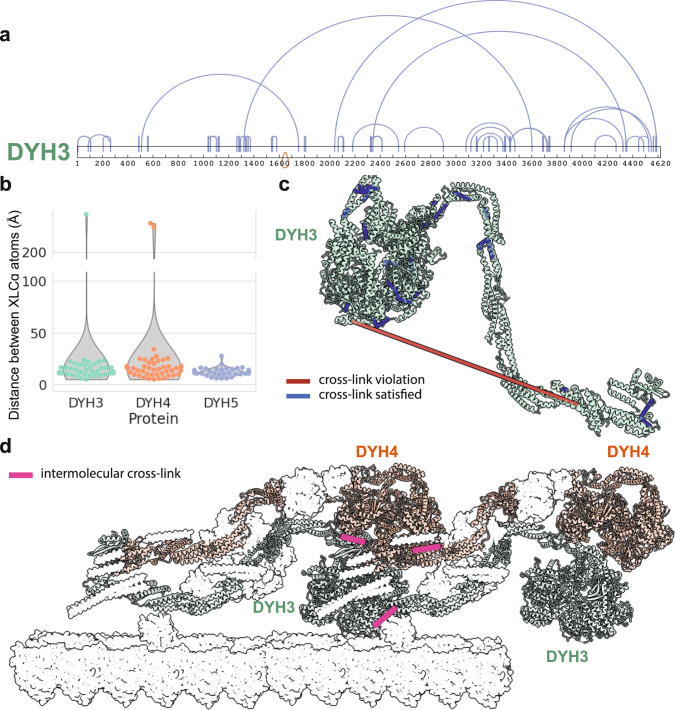
Fig. 2. In situ cross-links agree with the known T. thermophila outer dynein arm cryo-EM structure.
a Cross-link diagram for DYH3 shows the abundance of intramolecular cross-links within the protein. b We observed a total of 155 intramolecular cross-links across all three dynein heavy chain proteins, 124 of which corresponded to structured regions and hence could be used as a validation set. Intramolecular distances are plotted for these 124 cross-links. c Intramolecular cross-links mapped onto the DYH3 structure. In summary, there was a 97% agreement between cross-links and cryo-EM structures of the dynein proteins. d In situ assembly of ODAs, show that perceived monomer cross-link violations are actually satisfied between copies of dynein proteins, improving the cross-link agreement to 99% (PDB ID:7MOQ) (see also Supplementary Table S1 for specific values).