Abstract
Aims
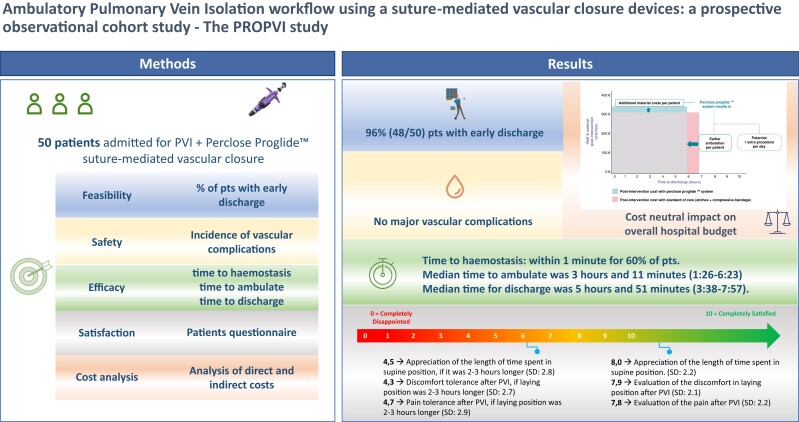
The leading reason for delayed discharge after pulmonary vein isolation (PVI) is vascular complications. This study aimed to evaluate feasibility, safety, and efficacy of the Perclose Proglide™ suture-mediated vascular closure in ambulatory PVI, report complications, patient satisfaction, and cost of this approach.
Methods and results
Patients scheduled for PVI were enrolled prospectively in an observational design. Feasibility was assessed as % discharged the day of procedure. Efficacy was analysed as acute access site closure rate, time to reach haemostasis, time to ambulate, and time to discharge. Safety analysis consisted of vascular complications at 30 days. Cost analysis was reported using direct and indirect cost analysis. A 1:1 propensity matched control cohort was used for comparing time to discharge to usual workflow. Of 50 enrolled patients, 96% were discharged on the same day. 100% of devices were successfully deployed. Immediate (<1 min) haemostasis was reached in 30 patients (62.5%). Mean time to discharge was 5:48 ± 1:03 h (vs. 10:16 ± 1:21 h in the matched cohort, P < 0.0001). Patients reported high level of satisfaction with the post-operative time. No major vascular complication occurred. Cost analysis showed a neutral impact compared to the standard of care.
Conclusion
The use of the closure device for femoral venous access after PVI led to safe discharge of patients within 6 h from the intervention in 96% of the population. This approach could minimize the overcrowding of healthcare facilities. The gain in post-operative recovery time improved patients’ satisfaction and balanced the economic cost of the device.
Keywords: Atrial fibrillation, Pulmonary vein isolation, Femoral access, Perclose ProGlide™, Ambulatory management, Rapid discharge
Graphical Abstract
Graphical Abstract.
What’s new?
There is still lack of knowledge on the use of vascular devices in the EP field; this paper provides evidence supporting the use of Perclose ProGlide™ suture-mediated vascular closure in PVI procedures.
A shorter post-operative supine position was preferred by patients.
Analysis of costs showed a neutral economical impact of vascular closure device use in PVI.
Introduction
Pulmonary vein isolation (PVI) for the treatment of atrial fibrillation (AF) is an increasingly performed procedure worldwide.1 The procedure workflow has been improving quickly through both the implementation of new ablative technologies and peri-procedural management, so that PVI is now routinely performed within 120 min.2 Although procedure time has been shortened significantly, the post-operative management of patients undergoing PVI has remained almost unchanged.3 Thus, the possibility to perform these procedures in a same day discharge setting presents an interesting prospect in the electrophysiology (EP) field. To date, the possibility of a rapid discharge after PVI is limited by post-procedural adverse events, driven mainly by vascular complications.1,3 The incidence of serious complications such as femoral pseudoaneurysm, arteriovenous fistula, and retroperitoneal bleeding are approximately 1.5% and increase with the number and size of sheaths used.1,3 In addition, the intensive peri-procedural anticoagulative regimen recommended at the time of PVI procedures aggravates the incidence of minor complication such as bleeding or haematomas.4 In order to reduce complications related to the venous puncture, an ultrasound approach for venous accesses has been adopted, resulting in a drastic decline in puncture-related complications.5–7 As for the post-operative management, a figure-of-eight suture and/or manual compression (MC), followed by post-operative bedrest (up to 8 h) is still the standard approach in many centres.5–7 Hence, the application of devices that can reduce the bedrest time after EP procedure is an interesting clinical opportunity.8–10 The aim of this study was to evaluate the feasibility, safety, and efficacy of using Perclose ProGlide™ suture-mediated vascular closure in percutaneous PVI procedures for the purpose of enabling a workflow without any bedrest after recovery from anesthesia. We evaluated the feasibility of this method to achieve early mobilization of patients undergoing PVI. We also investigated patients’ clinical symptoms and satisfaction. Incidence of vascular complication at Days 1, 7, and 30 following the intervention were analysed. Hospital costs compared to the standard of care (SOC) in our hospital was assessed considering direct and indirect costs.
Methods
Study design
We performed an observational prospective single-centre cohort study of patients admitted for PVI with Perclose ProGlide™ system use, from January 2020 to May 2021.
Data were prospectively collected; an electronic case report form (e-CRF) was promptly completed. Source data and database quality control was performed by investigators. A detailed description of the study design had been previously accepted by the local ethical committee. A Clinical Events Committee was recruited for the follow-up clinical events evaluation.
Variables and definitions
The primary endpoint (feasibility of an ambulatory PVI strategy) was assessed as the percentage of patients being able to be discharged the same day of the procedure. The secondary endpoints were analysed only for patients who met the primary endpoint. Acute vascular device closure performance was evaluated as the number of successful deployments out of total number of devices utilized (two for single PVI). Immediate haemostasis (< 1 min from device deployment) rate was recorded as a proportion of the total number of procedures. Post-procedural time to reach haemostasis was measured as the time from the delivery of the closure device to confirmed venous haemostasis in those patients needing further manual compression after device deployment. Time to ambulation was analysed as time from the removal of closure device to patients’ ability to walk. Time to be deemed suitable for discharge was calculated as the time from the removal of closure device to the medical assessment that deemed discharge possible. Time to discharge was considered as the effective time from the removal of closure device to patient discharge. Patient satisfaction was evaluated using the Post Ablation Procedure Patient Survey (see Supplementary material online). Minor and major vascular complications were calculated as the number of patients with venous access-related issues both requiring or not investigation, medical management, or surgical intervention out of the total number of patients enrolled.
Inclusion/exclusion criteria
All patients scheduled for elective PVI were considered eligible for study participation. All patients participating accepted to be enrolled in the study and signed the informed consent after detailed discussion. Exclusion criteria were: age <18 years, previous adverse event after vascular access resulting in prolonged hospitalization, previous vascular surgery in either leg or in the aorto-iliac axis, nonstandard ablation (i.e. need for more than two femoral punctures), known history of bleeding diathesis, coagulopathy, hypercoagulability or platelet count < 100 000 cells/mm3, history of deep vein thrombosis (DVT), pulmonary embolism or thrombophlebitis, significant anaemia or renal insufficiency, haemodynamic or electrical instability, body mass index (BMI) > 45 kg/m2 or < 20 kg/m2, active liver disease or hepatic dysfunction, severe renal dysfunction, defined as an estimated global filtration rate (eGFR) < 30 mL/min/1.73 m2 unless the patient is in renal support therapy.
Procedure
All subjects provided written informed consent prior to the PVI procedure. Patients were admitted to the hospital at the same day of the intervention. All procedures were performed under uninterrupted anticoagulation, if indicated, and heparin was administered during the procedure in order to get an activated clotting time (ACT) of 300 s or greater.1 No anticoagulation was given on the morning of the procedure itself. All procedures were performed under general anaesthesia. Two sheaths with 8 and 8.5 French diameter, respectively, were placed in the right common femoral vein via an US-guided approach. No local anaesthetic was given at any site during any phase of the procedure. At the end of the procedure, a Perclose ProglideTM-closure system was used for each sheath. After discharge from the anaesthesiology recovery unit, patients were kept in observation in an ambulatory recovery room, in a sedentary position without further bedrest, until discharge was deemed possible by the attending physician. A transthoracic ultrasound to exclude pericardial effusion was part of the pre-discharge assessment as per the standard of care for all patients in our institution.
Endpoint
The primary endpoint was the rate of patients discharged the same day of the procedure. Secondary endpoints were: (i) acute vascular device closure performance, (ii) post-procedural time to reach haemostasis, (iii) time to ambulation, (iv) time to possibility of discharge, (v) time to discharge, and (vi) patient satisfaction. Prior to discharge, all patients were asked to complete the Post Ablation Procedure Patient Survey questionnaire (see Supplementary material online) in which scores from zero (very unsatisfied) to 10 (very satisfied) were assigned. Level of pain, need for analgesic medications, and patient’s satisfaction were also evaluated using the ‘Post Ablation Procedure Patient Survey’ questionnaire (see Supplementary material online). The secondary safety endpoint was the incidence of minor and major vascular complications within 30 days after the procedure and according to the Clinical Event Committee (CEC) analysis.
Analysis of costs
Cost comparison considered direct and indirect costs including time and staff allocation spent in the EP lab and the ward. Procedure timings and relative costs were provided by the cardiology department of OLV Aalst. Costs related to nursing staff salaries are in accordance with the mandatory barema scales and expert opinion was sought for clinician staff costs. A detailed description of the process and methodology of the cost comparison is provided as a Supplementary material online to the manuscript.
Follow-up
Patients were followed-up for a period of 30 days after the procedure. Photographs of the puncture site were collected from the patients or their caregivers at Day 1 and/or Day 7 after the procedure. Patients were instructed to inform the investigators at any time with new cases of symptoms. A CEC consisting of three independent members evaluated the occurrence of adverse events.
Statistical analysis
For descriptive analysis, categorical variables are presented as absolute numbers and their relative percentages, continuous variables are presented as mean ± standard deviation (SD) and/or median and interquartile range (IQR) according to normal or non-normal distribution. Baseline characteristics are presented as numbers (%) for categorical variables and as means ± standard deviation for continuous variables. Differences between groups were analysed using the Student’s t-test or the Mann–Whitney U test for continuous variables, and the χ2 test or Fisher’s exact test for categorical variables, as appropriate. Propensity score (PS)-matching was used to reduce selection bias between the PROPVI group and the general population and to adjust for significant differences in the patients’ baseline characteristics. The propensity score was computed by a logistic regression model, and the matching was performed using the nearest neighbour method with a 1:1 ratio.
Matching criteria were age, sex, BMI, hypertension, diabetes, smoking habit, peripheral arteriopathy (PAD), and creatinine clearance. Analyses were performed with R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). A P-value of 0.05 was considered statistically significant.
Results
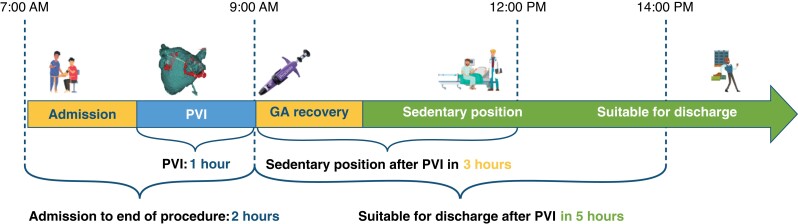
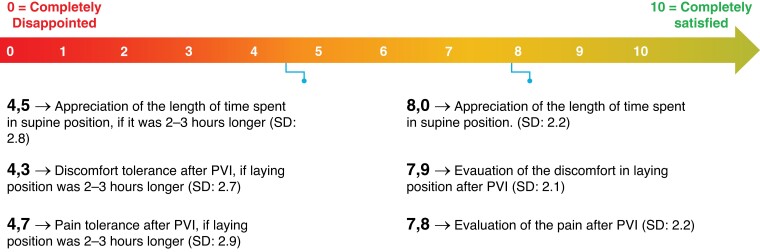
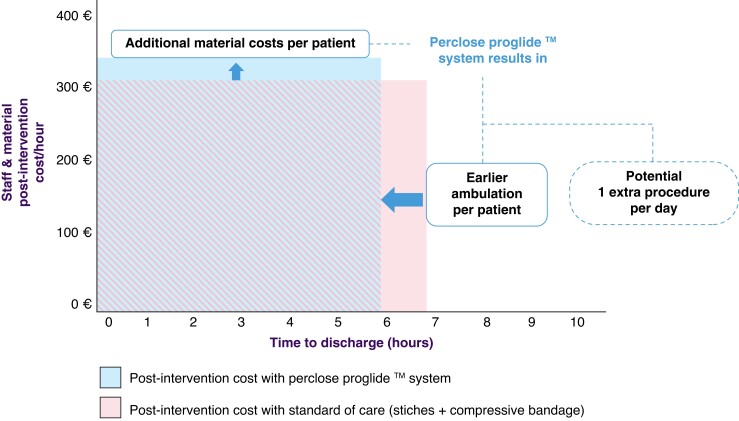
A predefined number of 50 patients were enrolled (Table 1). All patients underwent PVI with radiofrequency (RF) ablation. No intra-procedural complications occurred. At the end of the procedure, two Perclose ProglideTM systems were placed—one for each vein. In total 48/50 (96%) patients were discharged on the day of the procedure (Table 2). 49 patients (98%) were deemed to be suitable for discharge, but one patient requested to prolong the admission for non-medical reasons. One patient was kept supine due to discomfort until an ultrasound evaluation was carried the day after which excluded severe complications. Successful Proglide deployment was observed in all 48 patients that met the primary endpoint (96/96 devices, 100% success rate). After the procedure, anticoagulation was reversed by protamine administration in two patients (4%). Immediate haemostasis was reached in 30 patients (60%), with 20 subjects (40%) requiring short additional manual compression. In this last group, the mean and median time to achieve haemostasis were 4 min and 34 s (± 3:27) and 3 min (2–15), respectively. During the post-operative stay, two patients (4.2%) experienced minor bleeding, stopped after additional short manual compressions. Mean and median time to ambulation was 3 h and 18 min (± 1:05) and 3 h and 11 min (1:26–6:23), respectively. The mean and median time to discharge in the 48 patients was 5 h and 48 min (± 1:03) and 5 h and 51 min (3:38–7:57) (Figure 1 and Table 2). By using the ‘Post Ablation Procedure Patient Survey,’ patients reported high levels of satisfaction for the total duration of the supine position, as well as for discomfort and access-related pain during the post-operative supine position. The mean score assigned to ‘satisfaction about the total duration of lying position after PVI’ was 7.8/10, vs. a score of 4.7/10 that was assigned had a hypothetical supine position lasted 2–3 h longer. Similar results were observed regarding the discomfort and access-related pain during the post-operative supine position (Figure 2 and Table 3). Post-operative pain required management in only three patients, consisting of two single doses of salicylic acid or paracetamol and one repeated administration of paracetamol. There was no need for local analgesia or long-term analgesia prescription (Table 2). All patients concluded the 30-day follow-up period. They all received phone calls at Days 1, 7, and 30. No major vascular complications as well as necessity of any invasive intervention were described in the overall population (Table 2). Post-procedural haematomas bigger than 6 cm occurred in three patients (6.25%) which spontaneously resolved during the follow-up time with no further assessment or management needed. In 15 (31%) patient,s an asymptomatic superficial haematoma <6 cm occurred, and in one patient (2.08%) a transient access site-related nerve injury was reported (Table 2). For the analysis of cost, this workflow was compared to the figure-of-eight approach that represents the standard of care in our centre. With respect to the figure-of-eight method, the use of Perclose ProGlideTM increased the overall cost of the procedure (device and staff costs included) by 259,15€ favouring figure of eight closure. However, considering the time spent in the ward after the ablation, the use of Perclose ProGlideTM reduced the time to discharge by 60 min. This reduction potentially increases the cost effectiveness of the PVI unit by improving efficiency and flow-through of patients with the potential to increase the number of procedures being carried out (for example diuretic administration for treatment of heart failure). The above staff and procedure efficiencies offset the cost of the Perclose ProGlideTM system, resulting in a neutral economic impact for the hospital for a single patient (Figure 3).
Table 1.
Patient demographic, clinical, and procedural characteristics (overall population)
| Patient demographic, clinical and procedural characteristics | |
|---|---|
| Total population, n (%) | 50 (100) |
| Median age (IQr) | 64 (28–80) |
| Male sex, n (%) | 38 (76) |
| Median BMI (IQr) | 26.3 (21–42) |
| Hypertension, n (%) | 20 (40) |
| DM, n (%) | 11 (22) |
| Smokers, n (%) | 4 (8) |
| Necessity of protamine administration (%) | 2 (4) |
| Median ACT (s) | 318 (225–355) |
Table 2.
Endpoints and outcomes
| Total population, n (%) | 50 (100%) |
| Primary endpoint | |
| Discharge in the same day, n (%) | 48 (96)a |
| Outcomes | |
| Success of device deployment, n (%) | 96 (100) |
| Necessity of post-deployment manual compression > 1 min, n (%) | 20 (41.7) |
| Late recurrence of bleeding, n (%) | 2 (4.16) |
| Mean/median time to reach haemostasis (mm:ss)b | 4:35 (± 3:27)/3:00 (2:00–15:00) |
| Mean/median time to ambulation (hh:mm)—IQR | 3:18 (± 1:05)/3:11 (1:26–6:23) |
| Mean and median time to be deemed suitable for discharge | 4:55 (±00:54)/4:48 (2:50–7:30) |
| Mean/median post-procedural time to be discharged (hh:mm)—IQR | 5:48 (± 1:03)/5:51 (3:38–7:57) |
| Major vascular complications needing surgical intervention, n (%) | 0 (0) |
| Major vascular complications needing further medical evaluation/investigation, n (%) | 0 (0) |
| ȃHematoma > 6 cm, n (%) | 3 (6.25) |
| ȃAsymptomatic superficial bruising ≤ 6 cm, n (%) | 15 (31.25) |
| Necessity of pain medication, n (%) | 3 (6.25) |
| ȃParacetamol, n (%) | 2 (4.16) |
| ȃȃSingle administration, n (%) | 1 (2.08) |
| ȃȃRepeated administration, n (%) | 1 (2.08) |
| ȃSalicylic acid, n (%) | 1 (2.08) |
49 patients (98%) were deemed to be suitable for discharge. One of them wanted to prolong the hospital stay for non-medical reasons.
Described only for patients in which haemostasis within 1 min was not achieved.
Figure 1.
Patients’ flow in the PROPVI study. The timeline exposed in the figure is realized according to data from the population that reached the primary endpoint; to: admission in hospital; yellow arrow: waiting (pre and post) times, blue arrow: operative time; green arrow: endpoint GA = general anesthesia.
Figure 2.
Visual extract from the post ablation procedure patient survey. Patient’s preference for shorter supine position, less discomfort, and less pain. All three parameters can be achieved by using Perclose Proglide™ system.
Table 3.
Patients’ reported satisfactions after PVI
| Parameters of satisfaction | n | Mean | SD |
|---|---|---|---|
| Appreciation of the length of time spent in supine position | 47 | 8.0 | 2.2 |
| Discomfort laying position after PVI | 47 | 7.9 | 2.1 |
| Pain after PVI | 46 | 7.8 | 2.2 |
| Appreciation of the length of time spent in supine position if it was 2–3 h longer | 45 | 4.5 | 2.8 |
| Discomfort, if laying position was 2–3 h longer | 45 | 4.3 | 2.7 |
| Pain after PVI, if laying position was 2–3 h longer | 45 | 4.7 | 2.9 |
Patients were asked to assess satisfaction for each parameter in a range from 0 (very dissatisfied) to 10 (very satisfied).
SD, standard deviation; PVI, pulmonary vein isolation.
Figure 3.
Hospital efficiency gain. The increased efficiency gain when using Perclose Proglide™ system. Shaded are shows shared costs between both approaches. Despite the upfront incremental cost for Proglide (solid blue box), the system allows to mobilize the patient earlier (solid pink box), thus reducing the cost for the hospital.
From the contemporary cohort of patients undergoing PVI with standard-of-care treatment including figure of 8 suture, 166 patients were matched in a 1:1 ratio with the PROPVI study group. No differences were observed across baseline characteristics of the matched population (Table 4). Details of the propensity score matching are reported in Supplementary material online, Figures S1 and S2. Mean time to discharge for the control cohort was 10:16 ± 1:21 h (P < 0.0001 for comparison to the study group). Time to ambulation was not available for the control group as it does not apply to our standard figure of eight workflow.
Table 4.
Propensity matched population comparison
| Matched population (n = 50) | PROPVI population (n = 50) | Total population (n = 100) | P value | |
|---|---|---|---|---|
| Age mean (SD) | 59.9 (11.46) | 61.06 (11.71) | 60.48 (11.54) | 0.618 |
| Gender male n (%) | 38 (76%) | 38 (76%) | 76 (76%) | 1.000 |
| BMI mean (SD) | 27.70 (4.05) | 27.56 (4.95) | 27.63 (4.50) | 0.875 |
| Hypertension n (%) | 18 (36.0%) | 20 (40.0%) | 38 (38.0%) | 0.680 |
| Diabetes n (%) | 11 (22.0%) | 11 (22.0%) | 22(22.0%) | 1.000 |
| Smoke habit n (%) | 4 (8%) | 4 (8%) | 8 (8%) | 1.000 |
| PAD n (%) | 1 (2%) | 2 (4%) | 3 (3%) | 0.558 |
| Creatinine clearance Mean (SD) | 74 (25.7) | 81 (27.05) | 77(26.5) | 0.236 |
| hours for discharge Mean (SD) | 10.16 (1.21) | 5.48 (1.03) | 7.98 (1.12) | <0.001 |
| conversion to in hospital management n (%) | 4 (8%) | 1 (2%) | 5 (10%) | 0.1 |
Discussion
Although the PVI procedures have been shortened significantly over the last years, the post-operative management of patients undergoing PVI has remained almost unchanged normally requiring a post-operative bedrest up to 6 h and an intra-hospital observation up to 24 h.1,11 As a consequence, the number of PVI procedures performed is limited by the available bed capacity which was particularly affected in the era of restrictive rules applied for the COVID-19 pandemic containment.12 Thus, the possibility to perform PVI, and in general advanced procedures, in a day care setting, represents an interestingly prospect in the electrophysiology (EP) field, and emerging data support feasibility of same day discharge approaches using several ablation modalities.13,14 Existing data also support a potential for cost saving driven by a reduction in hospital costs, although there are little prospective controlled data available on this topic.15,16 The advantages of using devices for vascular closure have already been assessed in the context of arterial procedures.17 In particular, they were shown to reduce the rate of complication and the necessity of bedrest, shortening the total hospital stay.17 Despite the use of large femoral sheaths (up to 12 French) in the EP field, there is still a lack of knowledge about vascular closure devices. However, based on the low-pressure of the venous circulation, the effect of these systems can be even more advantageous than for arterial procedures. Recently, in a multicentre retrospective study investigating the usefulness of vascular closure devices after catheter ablation, a significant reduction of access-site complications, and ambulation time were observed.8 In another randomized multi-centre trial that assessed the use of the VASCADE MVP Venous Vascular Closure System, improvements were demonstrated with time to ambulation, total post-procedure time, time to discharge eligibility, time to haemostasis, and patient satisfaction.9 Our study demonstrates that an ambulatory strategy for pulmonary vein isolation procedure is feasible considering time to achieve haemostasis, time to ambulation, and time to discharge. This is of considerable importance for such increasingly rapid procedures, performed during intense anticoagulation and in an era favouring day case management. Since the majority of complications leading to prolonged hospitalization are related to the vascular approach, the use of a system able to properly close the femoral access is of critical importance to improve the femoral access management after the procedure. Furthermore, patient satisfaction with post-operative time was considerably higher than in the case of a postoperative stay longer than 2–3 h. In a time of shortage of healthcare workers and stress on hospital resources, the efficiency gain from medical technology innovations can be extremely beneficial. In our study, the Perclose ProGlideTM system, despite bringing an upfront incremental cost than the standard treatment, improves hospital efficiency and patient satisfaction. Reducing the ward stay and the staff costs, the Perclose ProglideTM closure approach offsets the cost of the Perclose ProGlideTM system, resulting in a final neutral impact for the hospital per single patient.
Limitations
There are several limitations in this study. The main limitation is the observational nature of the study design, making definitive comparisons with other established workflows impossible. However, to provide a basis for comparison, we performed a retrospective comparison with a propensity-matched cohort that can serve as a reference point for the time to discharge. Similarly, the analysis of costs for the comparator treatment is based on the price of the materials at the time the data were collected without setting a case control study. Also, patients were all managed with the Perclose ProglideTM closure device; thus, satisfaction analysis was based on a comparison on an only ‘virtual’ experience for patients.
We have chosen to only study uncomplicated PVI procedures in a first stage, excluding patients with higher probability of access related complications. Nevertheless, the stated exclusion criteria appear uncommon in straightforward PVI as zero patients scheduled for such procedures were ineligible for the study protocol.
Finally, the time to discharge in our study group is obviously the outcome of a micromanaged patient population and may very well represent the best achievable scenario. When comparing the time to discharge in the study group with the propensity-matched control group, it is important to keep in mind no particular efforts were made to optimize the time to discharge for the latter.
Regarding technical aspects of our workflow, we only assessed safety and feasibility using two venous sheaths with 8F diameter. We cannot extrapolate results to workflows using three or more sheaths. In addition, a two-sheath workflow where one sheath is large bore (e.g. cryoballoon) was not tested either. The authors do report anecdotal experience supporting feasibility of a three-sheath approach, and also report feasibility of large access closure if appropriate device recommendations are followed (specifically for the study device, the use of pre-close rather than post-close and the potential consideration of two devices per access site).
Conclusion
The PROPVI trial demonstrates that the ambulatory management of PVI by using the percutaneous Perclose ProglideTM closure device is safe and effective, appropriately closing venous accesses. The use of the closure device for PVI led to safe discharge of patients within 6 h from the intervention in 96% of the population with no major complications observed in the follow-up period. The ambulatory management described in the article is useful to reduce the post-PVI recovery time leading to a significantly improved patients’ experience. The cost of the Perclose ProGlideTM devices is balanced by savings made with the reduced use of day case department and by the decreased nursing time required. Further randomized trials are needed to better demonstrate the benefits of this approach.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors wish to express their explicit gratitude to Mrs Monika Beles, MSc, Mrs Giota Veliu, MSc, and Mrs Elitsa Stoyanova, PhD, for their assistance in manuscript preparation and their help in performing the cost assessment section. Giota Veliu is director Health Economics for Abbott EMEA and Elitsa Stoyanova is medical affairs liaison for Abbott EMEA. Monika Beles reports no disclosures.
Contributor Information
Davide Fabbricatore, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium; Department of Advanced Biomedical Sciences, University of Naples Federico II, corso Umberto I, 40, 80138 Napoli NA, Italy.
Dimitri Buytaert, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium.
Chiara Valeriano, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium; Department of Advanced Biomedical Sciences, University of Naples Federico II, corso Umberto I, 40, 80138 Napoli NA, Italy.
Niya Mileva, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium.
Pasquale Paolisso, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium; Department of Advanced Biomedical Sciences, University of Naples Federico II, corso Umberto I, 40, 80138 Napoli NA, Italy.
Sakura Nagumo, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium; Department of Cardiology, Showa University Fujigaoka Hospital, 1 Chome-30 Fujigaoka, Aoba Ward, Yokohama, Kanagawa 227-0043, Japan.
Daniel Munhoz, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium; Department of Advanced Biomedical Sciences, University of Naples Federico II, corso Umberto I, 40, 80138 Napoli NA, Italy.
Carlos Collet, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium.
Tom De Potter, Cardiovascular Center Aalst, OLV Hospital, Moorselbaan 164, 9300 Aalst, Belgium.
Funding
Study fundings were provided by Abbott Vascular International BVBA. T.D.P. and C.C. receive research grants and report consultancy fees from Abbott Vascular International BVBA. D.F. reports consultancy fees from Abbott Vascular International BVBA. D.F., P.P., and D.M. are supported by the Cardiopath PhD programme issued by the University of Naples Federico II in collaboration with the Cardiovascular Centre Aalst.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors’ contribution
T.D.P. conceived the study, designed the protocol, selected patients, and performed all the interventions, supervised the statistical analysis and the manuscript. D.F. performed the statistical analysis and wrote the manuscript. N.M. contributed to the manuscript. S.N. contributed to data collection. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Ethical approval
This trial has been previously evaluated and accepted by the institutional ethical committee.
Consent for publication
All patients were informed and signed a written informed consent for the publication.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reddy VY, Grimaldi M, De PT, Vijgen JM, Bulava A, Duytschaever MF et al. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol 2019;5:778–86. [DOI] [PubMed] [Google Scholar]
- 3. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111:1100–5. [DOI] [PubMed] [Google Scholar]
- 4. Gorla R, Dentali F, Crippa M, Marazzato J, di Minno MND, Grandi AM et al. Perioperative safety and efficacy of different anticoagulation strategies with direct oral anticoagulants in pulmonary vein isolation: a meta-analysis. JACC Clin Electrophysiol 2018;4:794–806. [DOI] [PubMed] [Google Scholar]
- 5. Okada M, Inoue K, Tanaka K, Ninomiya Y, Hirao Y, Oka T et al. Efficacy and safety of figure-of-eight suture for hemostasis after radiofrequency catheter ablation for atrial fibrillation. Circ J 2018;82:956–64. [DOI] [PubMed] [Google Scholar]
- 6. Yorgun H, Canpolat U, Ates AH, Oksul M, Sener YZ, Akkaya F et al. Comparison of standard vs modified ‘figure-of-eight’ suture to achieve femoral venous hemostasis after cryoballoon based atrial fibrillation ablation. Pacing Clin Electrophysiol 2019;42:1175–82. [DOI] [PubMed] [Google Scholar]
- 7. Aytemir K, Canpolat U, Yorgun H, Evranos B, Kaya EB, Sahiner ML et al. Usefulness of ‘figure-of-eight’ suture to achieve haemostasis after removal of 15-French calibre femoral venous sheath in patients undergoing cryoablation. Europace 2016;18:1545–50. [DOI] [PubMed] [Google Scholar]
- 8. Mohanty S, Trivedi C, Beheiry S, Al-Ahmad A, Horton R, della Rocca DG et al. Venous access-site closure with vascular closure device vs. manual compression in patients undergoing catheter ablation or left atrial appendage occlusion under uninterrupted anticoagulation: a multicentre experience on efficacy and complications. Europace 2019;21:1048–54. [DOI] [PubMed] [Google Scholar]
- 9. Natale A, Mohanty S, Liu PY, Mittal S, Al-Ahmad A, de Lurgio DB et al. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures: the AMBULATE trial. JACC Clin Electrophysiol 2020;6:111–24. [DOI] [PubMed] [Google Scholar]
- 10. Ben-Dor I, Craig P, Torguson R, Rogers T, Buchanan KD, Pokharel S et al. Mynxgrip® vascular closure device versus manual compression for hemostasis of percutaneous transfemoral venous access closure: results from a prospective multicenter randomized study. Cardiovasc Revasc Med 2018;19:418–22. [DOI] [PubMed] [Google Scholar]
- 11. König S, Svetlosak M, Grabowski M, Duncker D, Nagy VK, Bogdan S et al. Utilization and perception of same-day discharge in electrophysiological procedures and device implantations: an EHRA survey. Europace 2021;23:149–56. [DOI] [PubMed] [Google Scholar]
- 12. Gawałko M, Duncker D, Manninger M, van der Velden RMJ, Hermans ANL, Verhaert DVM et al. The European TeleCheck-AF project on remote app-based management of atrial fibrillation during the COVID-19 pandemic: centre and patient experiences. Europace 2021;23:1003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deyell MW, Hoskin K, Forman J, Laksman ZW, Hawkins NM, Bennett MT et al. Same-day discharge for atrial fibrillation ablation: outcomes and impact of ablation modality. Europace 2023;25:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanagaratnam P, McCready J, Tayebjee M, Shepherd E, Sasikaran T, Todd D et al. Ablation versus anti-arrhythmic therapy for reducing all hospital episodes from recurrent atrial fibrillation: a prospective, randomized, multi-centre, open label trial. Europace 2022;6:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahashi Y, Kuno T, Tanaka Y, Passman R, Briasoulis A, Malik AH. The 30-day readmission rate of same-day discharge protocol following catheter ablation for atrial fibrillation: a propensity score-matched analysis from national readmission database. Europace 2022;24:755–61. [DOI] [PubMed] [Google Scholar]
- 16. Tang PT, Davies M, Bashir Y, Betts TR, Pedersen M, Rajappan K et al. Efficacy and safety of same-day discharge after atrial fibrillation ablation compared with post-procedural overnight stay: a systematic review and meta-analysis. Europace 2022;24:1569–84. [DOI] [PubMed] [Google Scholar]
- 17. Jiang J, Zou J, Ma H, Jiao Y, Yang H, Zhang X et al. Network meta-analysis of randomized trials on the safety of vascular closure devices for femoral arterial puncture site haemostasis. Sci Rep 2015;5:13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.