Abstract
Conduction system pacing (CSP) has emerged as a more physiological alternative to right ventricular pacing and is also being used in selected cases for cardiac resynchronization therapy. His bundle pacing was first introduced over two decades ago and its use has risen over the last five years with the advent of tools which have facilitated implantation. Left bundle branch area pacing is more recent but its adoption is growing fast due to a wider target area and excellent electrical parameters. Nevertheless, as with any intervention, proper technique is a prerequisite for safe and effective delivery of therapy. This document aims to standardize the procedure and to provide a framework for physicians who wish to start CSP implantation, or who wish to improve their technique.
Keywords: Conduction system pacing, His bundle pacing, Left bundle branch area pacing, Device implantation
Introduction
Implantation of conduction system pacing (CSP) has risen dramatically and is gaining mainstream practice in tertiary centres across Europe. Data on the efficacy and safety of CSP are growing fast, and His bundle pacing (HBP) has been introduced in the European Society of Cardiology (ESC) supra-ventricular arrhythmia guidelines in 20191 and in the ESC pacing guidelines in 2021.2 A number of review articles on how to implant CSP have been written by early adopters of HBP and left bundle branch area pacing (LBBAP), but there is currently no consensus on which technique to use for implantation and confirmation of conduction tissue capture.
As with the European Heart Rhythm Association (EHRA) consensus document and practical guide on standard pacemaker (PM) and implantable cardioverter defibrillator (ICD) implantation,3 this document aims to provide up-to-date guidance for optimal implantation technique of CSP based upon published evidence and expert consensus, in order to standardize the procedure, improve success rates, avoid complications, and ultimately to improve patient outcome. Programming of CSP is covered elsewhere.4–6
Statements were based upon strength of evidence and consensus was reached by voting with at least 80% agreement by the contributing authors—who all have years of first-hand experience with CSP implantation.
Definitions
CSP implies direct activation of the conduction system of the heart by the pacing stimulus. This can be accomplished by pacing at the level of His bundle or its major branches or their further ramifications including distal Purkinje fibres.
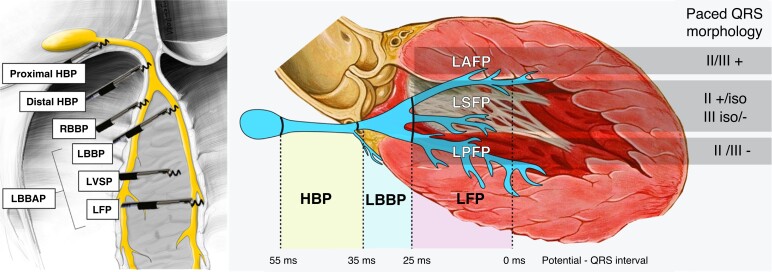
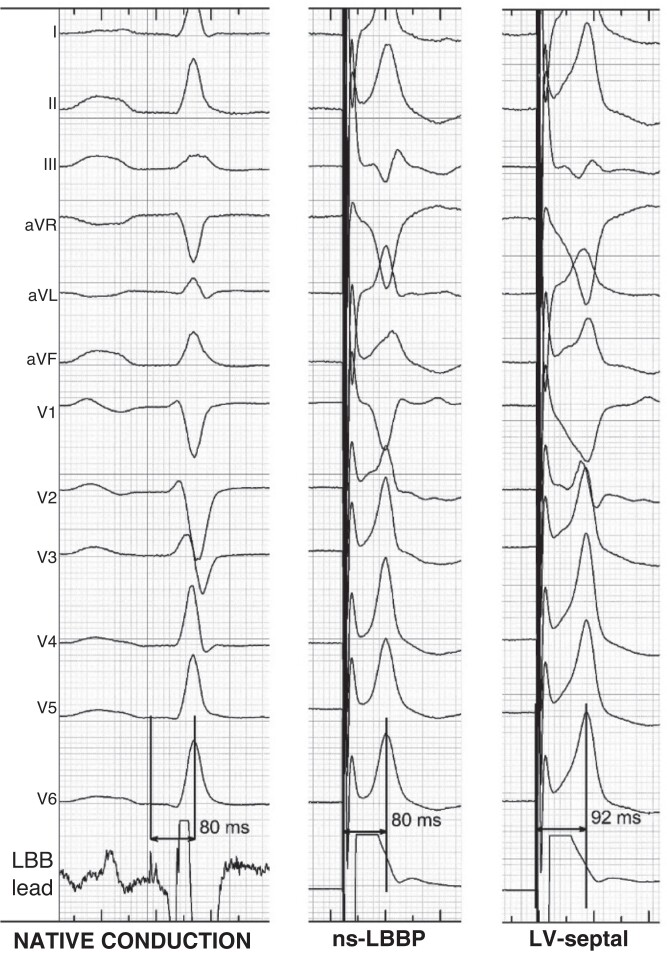
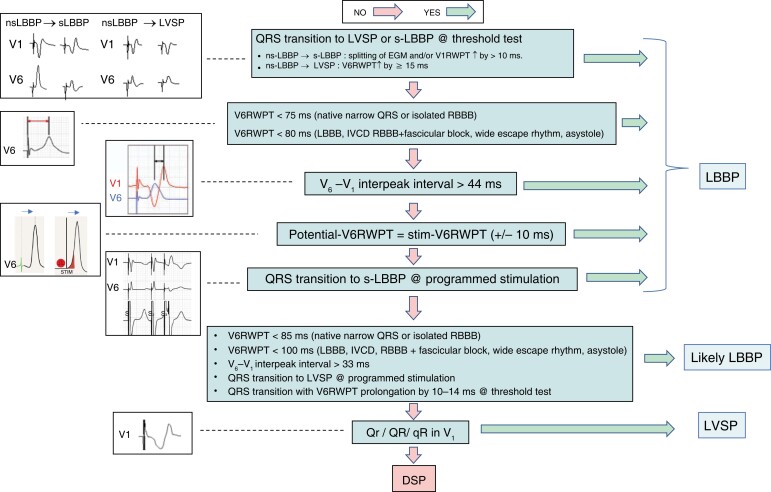
Determination of the level of conduction system capture rests on the anatomical position of the pacing lead, paced QRS morphology, and the potential to QRS interval (Figure 1). It is important to realize that these parameters have limitations. Fluoroscopic evaluation of the anatomical position may be imprecise, paced QRS morphology may be affected by myocardial substrate, conduction system potentials are not always visualized, or the potential to QRS interval may be lengthened by reduced conduction velocity of the diseased His–Purkinje system.
Figure 1.
Categories of conduction system pacing. Anatomical position of the pacing lead, potential to QRS interval (if visualized), and paced QRS morphology in leads II and III are used to determine the level of CSP. RBBP and LVSP are not shown on the right panel. HBP = His bundle pacing; iso = isoelectric; LAFP = left anterior fascicle pacing; LBBAP = left bundle branch area pacing; LBBP = left bundle branch pacing; LFP = left fascicular pacing; LPFP = left posterior fascicle pacing; LSFP = left septal fascicle pacing; LVSP = left ventricular septal pacing; RBBP = right bundle branch pacing. Modified with permission from Filip Plesinger and from Jastrzebski et al.7
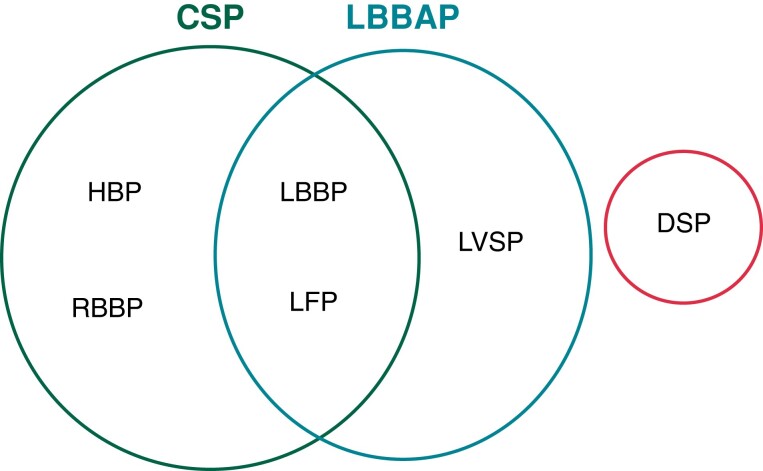
The different entities are defined below, and a synopsis is shown in Figure 2.
Figure 2.
Synopsis of different entities of CSP and related forms of stimulation.CSP = conduction system pacing; DSP = deep septal pacing; HBP = His bundle pacing; LBBAP = left bundle branch area pacing; LBPP = left bundle branch pacing; LFP = left fascicular pacing; LVSP = left ventricular septal pacing; RBBP = right bundle branch pacing.
His bundle pacing
His bundle pacing is defined as capture of the atrioventricular bundle with direct activation of all of its fibres. This part of the conduction axis of the heart is demarcated proximally by the distal atrioventricular node and distally by the division of the His bundle into the right bundle branch (RBB) and left bundle branch (LBB).
HBP is characterized by the pacing lead positioned near the tricuspid valve summit, either on the atrial or on the ventricular side of the tricuspid annulus, by the His bundle potential to QRS interval at the pacing site being ≥ 35 ms and the presence of HBP capture criteria.8 In some patients, the distal His bundle may be paced from deep within the septum.9
Right bundle branch pacing
Right bundle branch pacing (RBBP) may be encountered when the implanting physician intends to target the distal His bundle.10 RBBP is characterized by criteria outlined in Table 1.
Table 1.
Criteria for RBBP
| (1) Pacing lead in the distal His bundle region |
| (2) Evidence of conduction system capture (transitions in QRS morphology or selective conduction system capture) |
| (3) Paced V6RWPT > potential to V6RWPT (Δ > 10 ms) |
| (4) Additional findings which may be present: (a) Potential—QRS onset interval < 35 ms (b) Double transition in QRS morphology during threshold testing (requires different thresholds of the tissues): ȃns-HBP → ns-RBBP → RVSP → loss of capture ȃns-HBP → ns-RBBP → s-RBBP → loss of capture ȃns-HBP → s-HBP → s-RBBP → loss of capture (c) Selective paced QRS morphology ≠ intrinsic QRS morphology (unless baseline LBBB or RBBB) d) Paced latency > potential to QRS onset interval (requires selective capture) e) Δ V6RWPT ≤ 20 ms with loss of conduction system capture |
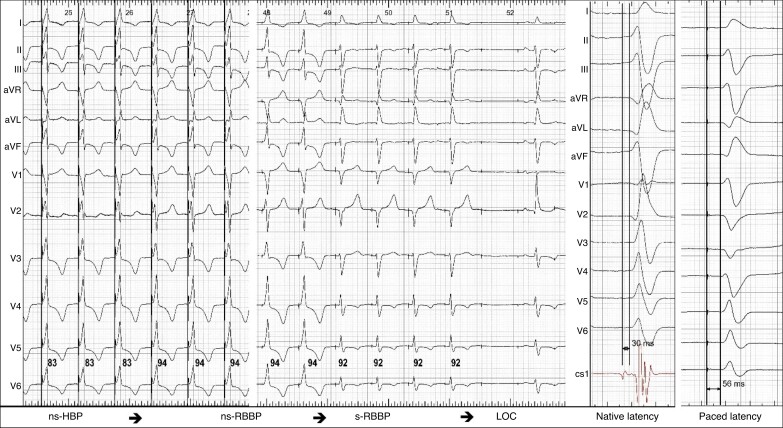
At working output, RBBP paced QRS is always non-selective, broader with a prominent pseudo-delta wave, and has longer V6 R-wave peak time (RWPT) than that during HBP, from which it may sometimes transition during threshold testing (see Figure 3). Confirmed capture of the distal RBB by a septal RV lead is rare.11
Figure 3.
Proximal RBBP with double QRS transition during threshold test. V6 R-wave peak times (V6RWPT) in milliseconds are indicated by the numbers and transitions are shown by the arrows at the bottom of the figure. Non-selective His bundle pacing (ns-HBP) transitions to non-selective right bundle branch pacing (ns-RBBP). Prolongation of V6RWPT by 11 ms (there is also a slight change in overall QRS morphology—see lead V2—illustrating the importance of recording a 12-lead ECG during threshold testing of CSP). Second transition is from ns-RBBP to selective (s-) RBBP. Note the change in the QRS axis with loss of myocardial capture. Pseudo-shortening of V6RWPT by 2 ms is related to change of R morphology to rS morphology in V6. The RBB potential to QRS interval of 30 ms recorded at the pacing lead implantation site is much shorter than latency interval during s-RBBP with a stimulus to QRS interval of 66 ms (not shown). Likewise, the potential to V6RWPT of 68 ms (not shown) is significantly shorter than the V6RWPT of 92 ms during s-RBBP. Moreover, s-RBBP QRS morphology is different from native QRS (most readily visible in V1–V3).
Left bundle branch pacing
Left bundle branch pacing (LBBP) is defined as capture of the pre-divisional LBB with simultaneous activation of all of its fascicles. This part of the conduction axis is demarcated proximally by the branching of the His bundle and distally by the first division of the main LBB.
LBBP is characterized by lead position deep in the interventricular septum, ∼1–2 cm from the distal His bundle potential (or the tricuspid valve summit), LBB potential to QRS interval in the range of 34–25 ms, normal QRS axis, and fulfilled criteria for conduction system capture (discussed later). In the MELOS registry reporting 2533 patients with LBBAP from 14 European centres, LBBP was encountered in only 9% of the patient cohort.7
Left fascicular pacing
Left fascicular pacing (LFP) is defined by the capture of one of the LBB fascicles or its distal arborization; this part of the conduction system is demarcated proximally by the ramification of the LBB and distally by the Purkinje fibres to myocardium junction.
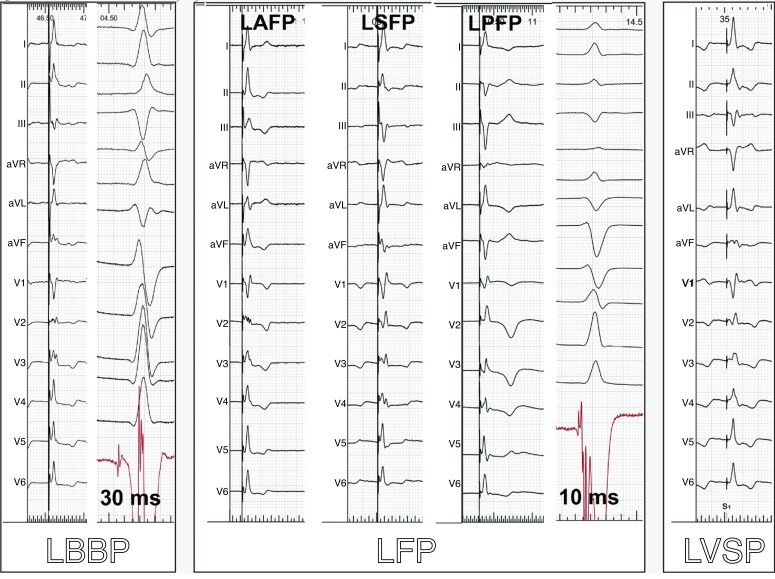
LFP is characterized by short potential to QRS interval (<25 ms), often with an abnormal paced QRS axis (usually superior and different compared with the native QRS axis) with the presence of criteria for conduction system capture.7 LFP is usually obtained when the pacing site is slightly more distant from the His bundle area (2–4 cm) than during LBBP. However, even a pacing site apparently quite close to the His bundle area can result in LFP due to substantial variability in LBB anatomy (early branching, fan-like ribbon shape, and possibly with sparse transverse interconnections).12 LFP is characterized by a relatively wide implantation target on the mid-septum and minimal pseudo-delta wave during non-selective pacing resulting often in a very narrow QRS. LFP may be further defined as being (i) left anterior fascicular pacing (LAFP) with positive QRS in leads II and III, (ii) left mid-septal fascicular pacing (LSFP) with positive/isoelectric QRS polarity in lead II and isoelectric/negative QRS polarity in III, and (iii) left posterior fascicular pacing (LPFP) with negative QRS in II and III. In the MELOS registry, LFP was the dominant form of LBBAP and was encountered in 69.5% of participants (17.2% LAFP, 27.5% LSFP, and 24.8% LPFP).7 Examples are shown in Figure 4.
Figure 4.
ECGs illustrating LBBP, LFP, and LVSP. QRS axis results both from conduction system capture and surrounding myocardial capture. Note the similarity in morphology between LBBP and left LAFP, which may be distinguished by the potential to QRS interval (if present) and anatomic lead position. The potential to QRS intervals recorded in the intrinsic rhythm by the pacing lead is also shown for LBBP and LFP. Sweep speed of 25 mm/s for the paced QRS tracings and 100 mm/s for those in intrinsic rhythm. Abbreviations as for Figure 1. Modified with permission from Jastrzebski et al.7
Left ventricular septal pacing
Left ventricular septal pacing (LVSP) is defined as capture of the left side of the interventricular septum without direct activation of the left conduction system. LVSP is characterized by terminal R-wave in V1, deep septal position of the pacing lead in the basal to mid-septal area, and absence of criteria for conduction system capture. During LVSP, the left-sided conduction system may be engaged retrogradely despite the lack of direct capture. In the MELOS registry, 21.5% of patients had LVSP.7
Left bundle branch area pacing
LBBAP is defined as capture of the subendocardial area on the left side of the interventricular septum,13 with or without simultaneous conduction system capture, and includes LBBP, LFP, and LVSP. This term rests on the anatomical lead position and QRS characteristics (terminal R-wave in lead V1—although in some cases, this may be absent). LBBAP is a practical designation intended to reflect the common scenario when differentiation between LBBP/LFP/LVSP is impossible, uncertain, or not feasible. Such situations can result from equal capture thresholds or similar refractoriness between conduction tissue and myocardium and/or nearly identical paced QRS morphology between LVSP and LBBP/LFP.
Deep septal pacing
Deep septal pacing (DSP) indicates that the pacing lead is positioned deep in the septum but does not reach the left ventricular subendocardial area. DSP is characterized by paced QRS narrower than that during right ventricular septal pacing and without notches in the left lateral leads. DSP is differentiated from LBBAP by the lack of terminal R-wave in lead V1 and no features of left conduction system capture.
This type of pacing often results from the inability to progress with the pacing lead to the left side of the septum. Nevertheless, it offers faster engagement of the left conduction system than RVSP. DSP should be differentiated from the occasional LBBAP cases without terminal R-wave in V1 due to the relatively early activation of the right ventricle.
Selective vs. non-selective capture
The terms selective vs. non-selective CSP refer to the absence or presence of the simultaneous capture of the ventricular myocardium adjacent to the conduction system.14
Non-selective (ns-) HBP is characterized by depolarization starting immediately after the pacing stimulus and can be recognized by the presence of a pseudo-delta wave in the surface electrogram and/or local potential fused with the pacing stimulus in the endocardial electrogram. Selective (s-) HBP is characterized by myocardial depolarization occurring after some latency period and can be diagnosed when there is an isoelectric interval in the surface ECG in all 12 leads and/or discrete potential after the pacing stimulus in the endocardial electrogram.5,8,15 Latency interval during s-HBP corresponds to the HV interval in endocardial electrogram during intrinsic activation, and QRS width should be measured from QRS onset. Left ventricular synchrony and function seem to be comparable between s-HBP and ns-HBP,16–18 as is the clinical outcome.19 However, ns-HBP is deemed safer, due to the presence of back-up ventricular capture, and sensing parameters are usually superior.17 More detailed ECG analysis of HBP is covered elsewhere.8
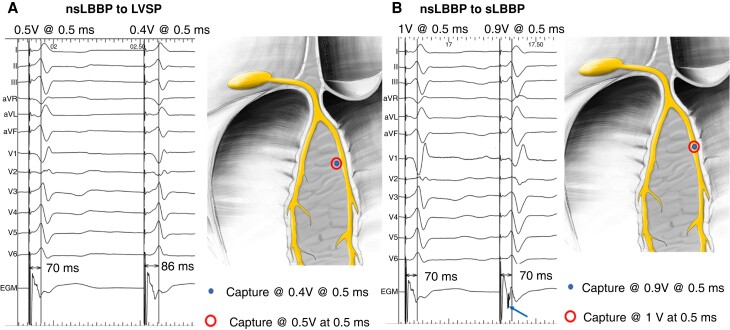
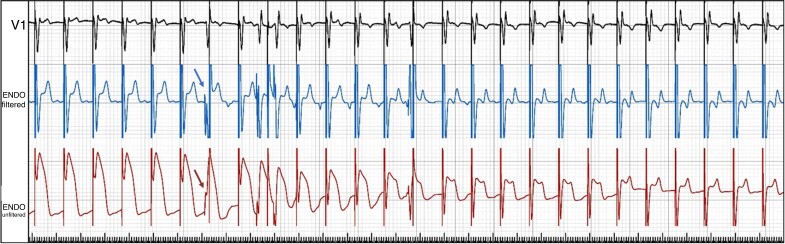
With LBBP, there often appears to be an isoelectric interval in all 12 leads at 100 mm/s display, even with ns-LBBP. By convention, the QRS width should be measured from the pacing spike.20 At working output (2.5 V/0.4 ms), ns-LBBP is almost always present. Transition from ns-LBBP to s-LBBP is mainly observed shortly after lead fixation, when the myocardial threshold is initially elevated due to the current of injury (COI), with equalization of myocardial and conduction tissue thresholds thereafter (and is therefore rarely observed at follow-up). It can be recognized by QRS widening and in V1 a ‘rounded’ appearance of the terminal R-wave and prolongation of the V1RWPT and a deeper S-wave in leads I/V6 (see Figure 22B). Splitting of the paced endocavitary electrogram is also observed (see Figures 5 and 22A).
Figure 22.
(A) Transition between ns-LBBP and myocardial capture only with LVSP at 0.5 V@0.5 ms. This type of transition is characterized by prolongation of the V6RWPT, terminal R, or r amplitude decrease in V1 and usually by the disappearance of the S-wave in I/V6 during myocardial capture, but without a significant change in the local ventricular EGM signal. (B) A transition between ns-LBBP and s-LBBP at 1 V@0.5 ms is shown. This type of transition is characterized by prolongation of the QRS (measured from the pacing artefact). Usually, there is a change in QRS morphology from QR/qR to rSR in V1 (with rounding of the R’) and the development of a deeper S in I/V6 during s-LBBP, without change in V6RWPT. In the ventricular EGM signal, discrete local ventricular potential appears during s-LBBP (blue arrow)—indicating that local myocardium was no longer captured. Modified, with permission by Filip Plesinger.
Figure 5.
(A) Connection setup for CSP (modified, with permission, from Medtronic). (B) Screen setup with the 12-lead ECG and intracardiac electrogram recorded at 100 mm/s sweep speed during LBBAP implantation, with a filtered 30–500 Hz channel (cyan) and unfiltered 0.5–500 Hz channel (green). V1 and V6 are coloured in yellow to be distinguished from the other leads to facilitate analysis in real time. Note the COI in the unfiltered channel, and transition from non-selective to selective LBBP (last two cycles) with changes in ECG and EGM morphology. CSP F = conduction system pacing, filtered; CSP NF = conduction system pacing, non-filtered.
General considerations
Training
Even experienced device implanters encounter a learning curve when they begin implanting conduction system leads, with flattening of the fluoroscopy duration after 30–50 cases for HBP7,21 and after 110 cases for LBBP.7 We therefore recommend that new implanters obtain training in CSP through proctoring or attending dedicated courses. It is also important that allied professionals are properly trained for assisting with the procedure to ensure a successful programme.
Cardiac catheter laboratory setup
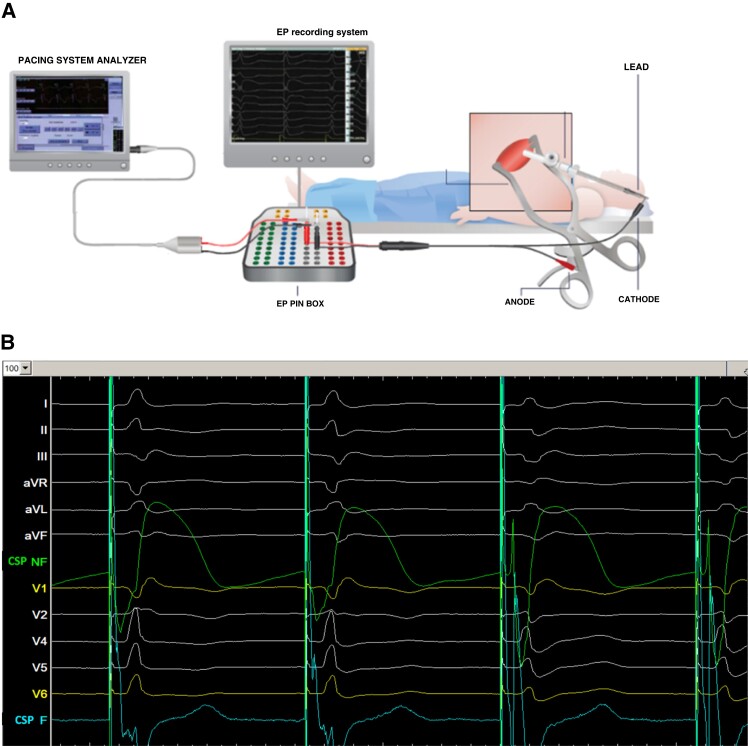
A 12-lead ECG is mandatory to assess paced QRS morphology for determining pacing location and confirming conduction tissue capture. Displaying real-time electrogram signals recorded from the pacing lead is also necessary for visualizing endocardial signals.
The ECG and lead electrograms are best displayed using an EP recording system, with the signal from the lead sent to both the pacing system analyser (PSA) and EP system using a Y connector or jump cables (see Figure 5).
It is strongly recommended to display both filtered (30–500 Hz) and unfiltered (0.5–500 Hz) signals. The latter allows visualization of COI. CSP COI impacts acute and long-term capture thresholds22,23 while myocardial COI is important for assessing tissue contact and monitoring lead depth to diagnose septal perforation.13,24
Filtered signals at gain settings used for visualizing conduction tissue potentials may be clipped. The myocardial COI is therefore more readily monitored on a separate unfiltered channel with an adjusted gain setting which shows the entire ventricular electrogram, or on a PSA. The sweep speed should be set to 50–100 mm/s in order to better distinguish conduction system potentials from the ventricular potential. Pacing impedance is measured either via the pacing unit from the EP recording system or via the PSA, and capture thresholds are measured using the PSA.
If an EP recording system is not available, endocardial signals recorded by the pacing lead may be displayed on a PSA, at a 0.05 mV/mm gain. In this instance, a 12-lead ECG will also be necessary to confirm conduction tissue capture but does not have the benefit of continuous recording of signals for review nor electronic callipers for precise measurement of timing intervals such as the RWPT. The 12-lead ECG and the PSA screen can be connected to external monitors to facilitate real-time assessment during procedure by the operator.
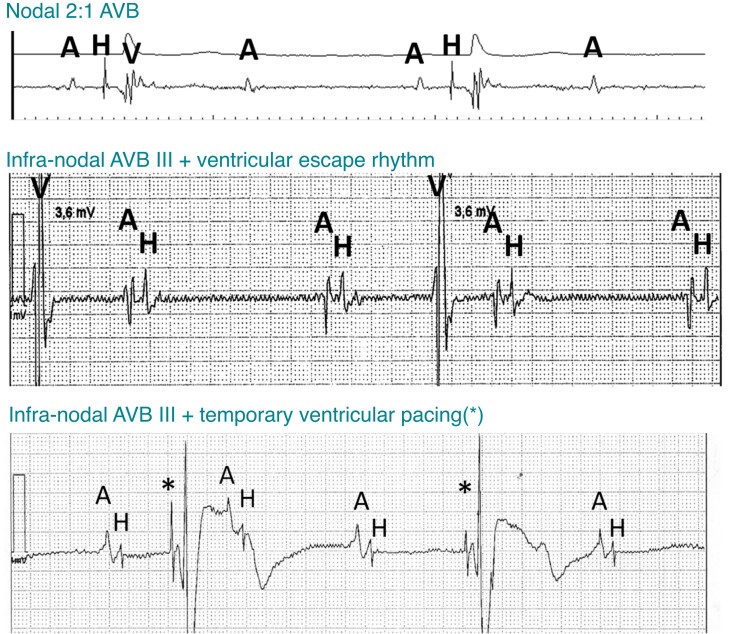
The implanting physician should be familiar with basic electrophysiology of the conduction system, especially the know-how to interpret endocardial signals for diagnosing nodal or infra-nodal block (Figure 6). Consideration should be given to placing a temporary back-up pacing lead in patients with a wide junctional escape rhythm or left bundle branch block (LBBB). If ICD lead implantation is planned in these patients, this should be performed first; if an atrial lead is to be implanted, it can be temporarily positioned in the RV.25 The back-up pacing lead should be connected to an additional PSA or external pacemaker while the CSP lead is being implanted.
Figure 6.
Examples of sinus rhythm with nodal (top panel) and infra-nodal block (middle and bottom panels). His potentials are only visible with infra-nodal block during blocked cycles. Pacing spikes (*) from a temporary lead may mimic His potentials. The two bottom tracings were recorded using a Merlin programmer (Abbott, Sylmar, CA, USA) with filtered (middle panel) and unfiltered (bottom panel) signals at a 0.05 mV/mm gain setting. A = atrial; AVB = atrioventricular block; H = His; V = ventricular.
Personnel
CSP implantation is greatly facilitated by having a member of the team who is able to perform the measurements and manoeuvres required to confirm conduction system capture. Prompt recognition of fixation beats, drop in impedance, or sudden decrease of myocardial COI is also facilitated by having a person dedicated to evaluating electrical measurements (as the operator may be focusing on fluoroscopy and handling of the lead).
His bundle pacing lead implantation
A recorded case of HBP implantation is available as video S1.
Locating the His bundle
The His bundle is made up of multiple fibres, which are predestined to become the RBB and posterior, septal, and anterior fascicles of the LBB.26 The His bundle measures approximately 20 mm in length and is encapsulated in fibrous tissue that insulates it from atrial and ventricular myocardia; therefore, mere recording of His bundle potential, even near-field, does not ensure capture. The His bundle begins from the AV nodal tissue and courses along the membranous septum in the right atrium before penetrating to the left side on the crest of the muscular portion of the interventricular septum. With lead placement in a proximal His position on the atrial aspect of the tricuspid annulus, s-HBP is frequently encountered, however sometimes with sensing issues (low R-wave amplitude, atrial/His oversensing); with more distal placement on the ventricular aspect of the annulus, ns-HBP is more frequent, with superior electrical parameters compared to proximal HBP.27–29 It is important to realize that a significant length of the proximal His bundle rests on the right atrial–left ventricular part of the membranous septum, which is above the tricuspid valve plane, meaning that non-selective His capture (i.e. including ventricular myocardial capture) may be observed on the atrial aspect of the tricuspid annulus.28 Conversely, selective His capture may be observed on the ventricular aspect of the tricuspid annulus in cases where the His bundle may be superficial and devoid of surrounding myocardial tissue.28
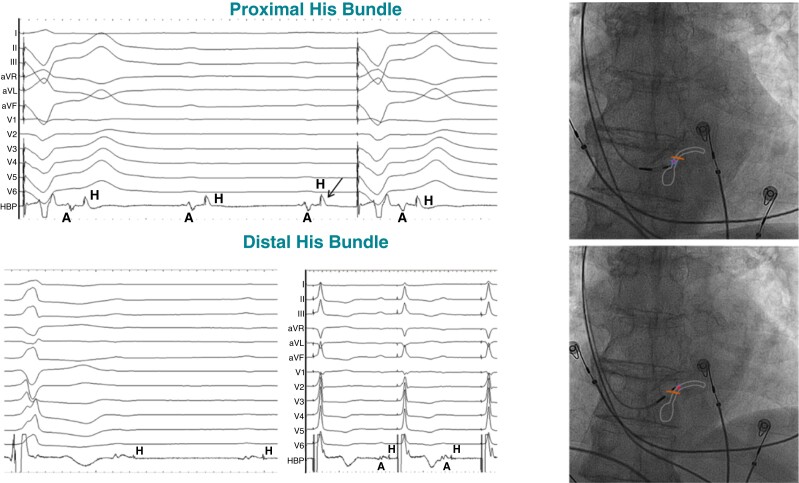
The His bundle can be readily mapped using the pacing lead in a unipolar fashion and dedicated electrophysiology catheters are usually not necessary. The pacing lead is typically delivered through a delivery sheath (fixed curve or steerable) with a posterior curve which directs the pacing lead to the septum. Once the sheath and lead are placed at the tricuspid annular region, mapping is performed in a 20–30° right anterior oblique (RAO) view for a site with a near-field (sharp) His signal with absent or small far-field atrial electrogram and a larger ventricular electrogram (Figure 7).
Figure 7.
Mapping the distal His bundle in a patient with infra-nodal block. Top panel: intracardiac electrograms from the HBP lead demonstrate a larger atrial electrogram (A) and prominent His (H) potential, where distal His capture could be achieved only at a high output due to HV block (despite COI of the His potential shown by the arrow). Ventricular pacing is delivered by an additional back-up lead. Bottom panel: at a slightly distal location, much smaller atrial electrogram is noted with His potential (likely far-field) where distal His capture is achieved at low output (1 V). Fluoroscopic images of the proximal and distal His positions of the HBP lead are shown along with schematic representation of the site of conduction block. Adapted with permission from Vijayaraman et al.30
If His bundle electrograms cannot be identified, pace mapping at high output (5 V@1 ms) can be performed as an alternative method to locate the His bundle, yielding narrow paced QRS complexes. This might be especially useful in patients with AV block and no escape rhythm.31
In patients with persistent atrial fibrillation, high-frequency atrial signals can mimic His electrograms. In these patients, it may be reasonable to begin mapping from the ventricular side of the high membranous septum. Additionally in patients with atrial fibrillation, it is critical to avoid a His location with large atrial electrograms (>0.5 mV), closer to a potential site of AV junction ablation to prevent rise in His capture thresholds if ablation is performed.32,33 While His electrograms can be easily located preceding the ventricular escape rhythm in patients with AV block at the nodal level, His electrograms often follow the atrial electrograms with short A–H intervals in patients with infra-nodal (HV) block.
Visualization of the tricuspid annulus and/or the triangle of Koch by injecting contrast at the atrial or ventricular side of the tricuspid valve via the delivery sheath has been proposed by some investigators to facilitate locating the His bundle (Figure 8 and video S2).34,35
Figure 8.
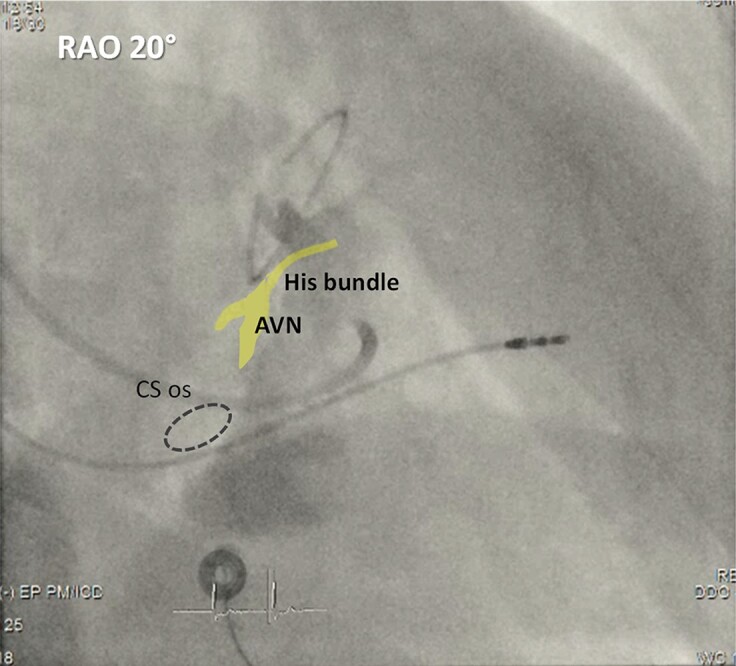
Contrast injection via a delivery sheath in a RAO view to delineate the tricuspid annulus. The atrioventricular node (AVN) and His bundle are depicted in yellow, and the coronary sinus ostium (CS os) is depicted by the dotted circle. The patient had prior mitral annuloplasty and VVI pacemaker implantation and underwent upgrade due to pacing-induced cardiomyopathy.
If the His region cannot be easily reached, the delivery sheath can be appropriately manually reshaped with the dilator in place (which avoids kinking the sheath). In patients with challenging anatomy, complex congenital heart disease, or pregnancy, 3D electroanatomical mapping may be helpful to facilitate mapping and locating the His bundle and/or to achieve HBP with minimal fluoroscopy.36–40 It may be more challenging to locate the His bundle with a right-sided implant, due to the curvature of the current guiding catheters being better suited for a left-sided approach, but this is nevertheless feasible.
With stylet-driven leads, mapping of the His bundle with a retracted helix can facilitate movement and reduce the possibility of helix entrapment in the chorda tendinea or the fibrous tissue surrounding the AV node but requires deploying the helix once the position is reached (which may result in an advertent modification in lead position).
Lead fixation
Lead fixation is a crucial part of the HBP procedure which determines acute and long-term electrical parameters as well as lead stability. The introduction of a fixed delivery catheter with a secondary posterior (septal) curve has improved electrical parameters and lead stability compared to placement using a two-dimensional deflectable sheath, thanks to more favourable orientation for lead fixation.41 Once the target zone has been identified, the delivery catheter must be continuously controlled by the operator and gently pushed in close contact with the cardiac tissue with a counter-clockwise rotation to stabilize the position while screwing the lead. Some forward force on the lead may be exerted as the lead body is rotated (without releasing the lead between turns) to facilitate penetration of the helix into the fibrous tissue surrounding the His bundle. The number of rotations which are necessary to fixate the lead is variable. Resistance to rotation and torque build-up rather than number of rotations indicates that the lead is being properly fixated. However, torque build-up may also result from resistance to lead rotations due to angulations of the delivery catheter and does not always imply tissue penetration. There must be an evident rebound of the lead when it is finally released. If this is not the case, rotations should be repeated, or the delivery catheter should be repositioned. At this stage, the presence of His bundle COI and/or change of His bundle potential polarity from positive to negative (often with some widening of the His bundle potential) should be assessed.22,42 If COI is absent, lead rotation should be repeated with the aim to create even a stronger torque in the lead than on the previous deployment attempt. Often after such manoeuvre, the COI and negative His bundle potential will appear—indicating that despite apparently good initial deployment, the contact between the pacing lead and His bundle was still inadequate, potentially compromising the long-term threshold stability (Figure 9). Once the lead is fixated, stability may be assessed by withdrawing the guiding catheter about 5–8 cm to expose the lead, which can be gently pulled or pushed to give a ‘U’ form with two additional rotations of the lead to test for changes in capture threshold or reduction of His potential amplitude.43
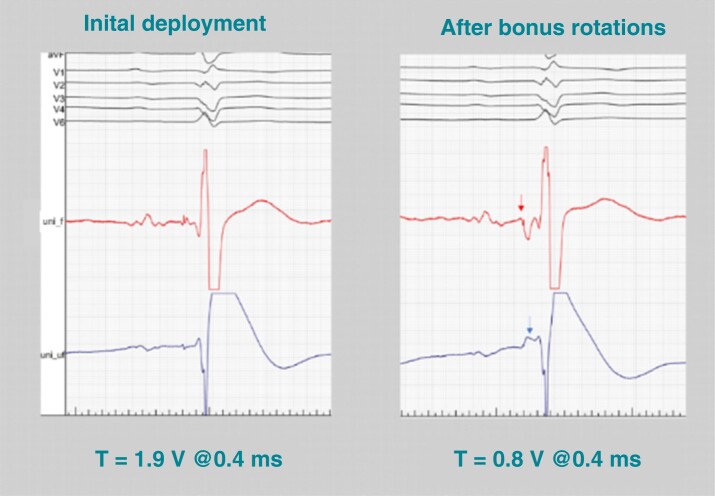
Figure 9.
His bundle COI as a marker guiding successful lead deployment. Despite apparently proper lead fixation with ≥5 lead rotations and acceptable acute threshold (left panel), the lead was not well deployed. After additional rotations, torque build-up was felt and a current of injury (blue arrow) and negative His potential (red arrow) were visible with a significant improvement of capture threshold (right panel). The lead was initially not in good contact with the His bundle and potentially unstable, thus increasing the risk of late threshold rise. Unipolar electrogram from pacing lead: uni_f—filters: 30–500 Hz; uni_uf—filters: 0.5–500 Hz.
Confirming His bundle capture and testing electrical parameters
The integral part of testing of electrical parameters is the assessment of paced 12-lead ECG and confirmation of His bundle capture which is covered in detail elsewhere.8,44,45 Briefly, sudden output-dependent transitions in QRS morphology during threshold testing serves as the key method to determine His bundle capture. Morphological features may also be useful to distinguish His bundle capture from myocardial capture (see Figure 10).46
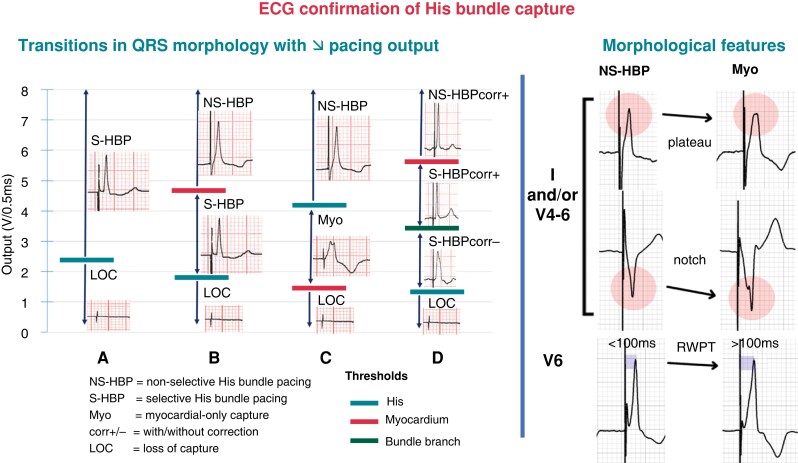
Figure 10.
ECG features to diagnose His bundle capture. Left panel: a number of different transitions in QRS morphology may be observed with decrementing pacing output, reflecting loss of His capture, myocardial capture, or correction of bundle branch block. The sequence of transition will depend upon the respective thresholds. (A) Obligatory S-HBP. (B) Transition from NS-HBP to S-HBP. (C) Transition from NS-HBP to myocardial capture only. (D) Transition from NS-HBP to S-HBP with and without correction of bundle branch block. Right panel: morphological features combining the absence of plateaus, notching, and/or slurring in leads I, V1, and V4 to V6 and V6 R-wave peak time (RWPT) < 100 ms indicate NS-HBP and allow to distinguish this entity from simple myocardial capture. Adapted with permission from Burri et al.8
Another method is to compare delays to the R-wave peak in V6, from the His potential in intrinsic rhythm and from the pacing stimulus during HBP. His capture is indicated by a difference of <12 ms, with an accuracy of 96.7%.47
Paced QRS with HB capture and features of the LBBB-like depolarization pattern (notch/slur/plateau in leads I, V4–V6, and stimulus-V6RWPT > 110 ms) indicate non-physiological LV activation and should not be accepted. If transitions are not observed, testing at shorter pulse widths (0.2–0.3 ms) may serve to enhance differences in threshold amplitudes due to divergence of the strength–duration curves of the His bundle and myocardium at shorter pulse widths (see Figure 11).48 In case of doubt, programmed pacing can be useful and exploits differences in refractoriness of conduction tissue and myocardium.49 With transitions from ns-HBP to s-HBP, the endocavitary electrogram typically shows separation with an isoelectric interval and change from an initial negative to a positive deflection15 similar to what is depicted in Figure 5B with LBBP.
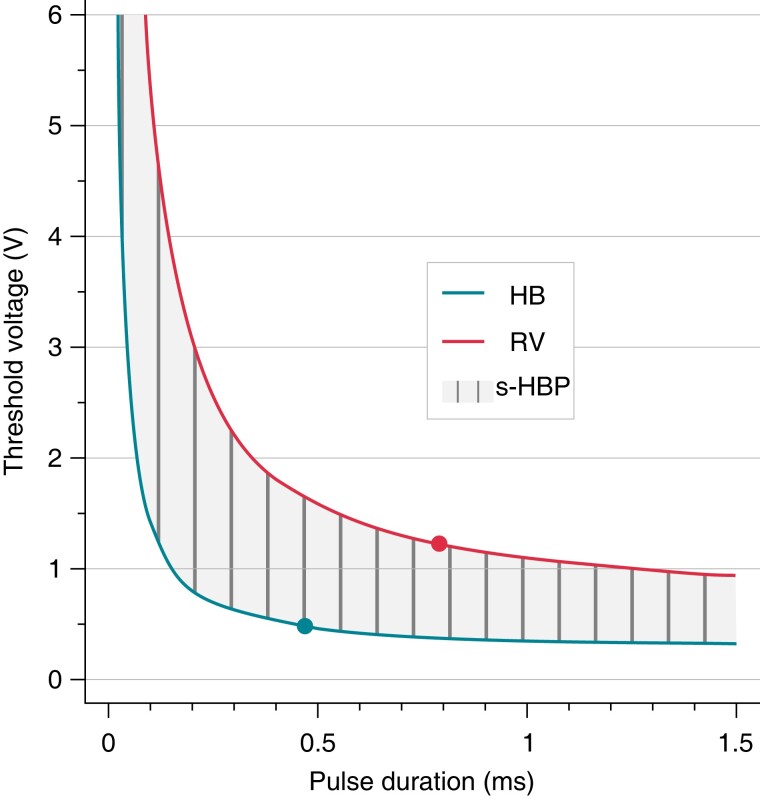
Figure 11.
Pacing chronaxie (dots) for His bundle (HB) is shorter than for right ventricular (RV) myocardium. Consequently, zone of selective pacing (s-HBP) widens with shortening of the pulse duration. By the same token, longer pulse duration facilitates simultaneous capture of both His bundle and RV myocardium. Adapted, with permission, from Jastrzebski et al.48
The pacing threshold of HBP should be measured at 0.5 ms and, if elevated, also at 1 ms. In addition, in case of bundle branch block, the threshold for correction should be reported. Thresholds ≤ 1.5 V/0.5 ms are optimal for HBP, but up to 2.0–2.5 V@0.5 ms may be acceptable in some clinical scenarios (e.g. heart failure, only option for electrical resynchronization). With ns-HBP, myocardial and His bundle thresholds should be reported separately. The presence of His bundle COI results in transient high His bundle capture threshold, which should be re-evaluated at five-minute intervals. In patients with long HV intervals or HV block, His capture with 1 : 1 HV conduction at cycle lengths ≤ 400 ms50 should be demonstrated at working output. Conduction block during incremental pacing manifests itself by loss of ventricular capture or by QRS prolongation with loss of His bundle capture criteria (e.g. development of notches or slurs). Such observations indicate that the HBP site is at or proximal to the lesion in the conduction system and a more distal site should be tried. HBP may result in atrial capture, which can result in a 1 : 1 ventricular response in case of preserved atrioventricular conduction and should also not be confounded with selective capture.
Capture thresholds and pacing impedances should be measured in the unipolar and bipolar modes, and after having withdrawn the guiding catheter by a few centimetres to avoid artificially lowering thresholds by improving lead contact. If non-selective capture is clinically desired (pacemaker-dependent patient, high HBP threshold), longer pulse width might be used (1.0–1.5 ms); if selective capture is desired (due to a broad ns-HBP QRS), shorter pulse width might be used (0.2–0.3 ms)—see Figure 11.
Sensing with HBP requires much more attention than with traditional RV pacing. Bipolar sensing amplitude should ideally be >2.0 mV (or unipolar > 4 mV). It is also important to carefully check for atrial or His bundle oversensing which may be fatal in patients with complete heart block. When sensing is inadequate, either a more distal His bundle site should be attempted or an additional RV back-up lead for sensing should be implanted. In patients with borderline values, it is necessary to repeat sensing tests with the lead connected to the device, as filters may differ from the PSA, yielding different values.
In addition to poor sensing by the HBP lead, a ventricular back-up lead should be considered in specific situations (e.g. pacemaker dependency, high-grade AVB, infra-nodal block, high pacing threshold, and planned AVJ ablation) according to the 2021 ESC pacing guidelines (class IIa, level of evidence C recommendations).2
Slitting
Before slitting the His bundle lead delivery catheter, the atrial lead and any other additional leads should be implanted to avoid dislodging the His lead and to provide additional waiting time before retesting the lead. It is important to maintain adequate slack within the cardiac chambers prior to and during sheath removal, to avoid inadvertent lead dislodgment. For slitting the sheath, the operator fixes the lead to the slitting tool, which is kept firmly in place while the other hand pulls back the catheter. If the lead is twisted upon itself after slitting (taking an α shape, usually observed with the 3830 lead), it should be carefully unravelled with a counter-clockwise rotation. Adequate slack, tested with deep breathing, should be given,51 and the lead tip should be stable without any rocking motion. The thresholds should be verified after slitting, once slack is adjusted.
Left bundle branch area pacing
Cases of LBBAP implantation using lumenless lead and stylet-driven lead (SDL) are shown in videos S3 and S4, respectively.
Localizing the lead insertion site
In contrast to HBP, where the His bundle can be directly targeted using activation mapping or pacemapping, the LV septal subendocardial course of the LBB implies that only indirect markers of LBB position can be used during the first steps of LBBAP lead implantation. The initially described technique for LBBAP lead positioning relies upon using distal His bundle potential as an anatomical marker and paced QRS morphology.52–58 First, the His bundle recording is localized with the pacing lead and the fluoroscopic location of the His bundle is tagged as a reference in the RAO view at 20–30°. The advantage of locating the His bundle is standardization and more accurate location of the reference point, evaluation of infra-nodal conduction, and evaluating if HBP is a feasible alternative to LBBAP. Drawbacks are extra time and fluoroscopy necessary to localize the His bundle potential and risk of RBB damage during His bundle mapping. Accordingly, a common modification of this technique is based on skipping His bundle mapping and using the tricuspid valve summit (which approximates the His bundle location) as an anatomical marker. Localization of the tricuspid summit can be done using contrast delivery through the guiding sheath, but this is rarely necessary. Observing the endocardial ‘A’ and ‘V’ potentials and sheath/lead movement, the superior part of the tricuspid annulus can be determined and then tagged to serve as a marker in the same fashion as the His bundle potential.
Next, the sheath is advanced ∼15–20 mm towards the RV apex, with the lead withdrawn within the sheath or the helix retracted with standard stylet-driven leads (SDLs) to avoid snagging the screw as the sheath crosses the tricuspid annulus. The electrode spacing of the lead gives an idea of distance (most leads have a spacing of ∼15 mm between the tip of the screw and the proximal part of the ring electrode). Counter-clockwise torque is then applied so that the sheath can reach the RV basal to mid-septum, where the LBB is expected to be located at the opposite site of the septum. At this point, the lead is exposed in contact with the right side of the interventricular septum and connected in a unipolar configuration to the PSA. The next step is to evaluate the paced QRS morphology obtained at this initial position. A good site for lead penetration often shows a ‘W’ pattern with a notch at the nadir of V1 QRS (although this is not mandatory) and discordant QRS in leads II (predominantly positive or equiphasic) and III (equiphasic or predominantly/completely negative). Examples are shown in Figure 12. Sites with concordant positive or negative QRS polarity in leads II and III can also result in successful CSP and correspond to LAFP or LPFP, respectively.7
Figure 12.
Localizing the initial lead deployment site on the septum with initial pacemapping of the right septum and final position after screwing the lead up to the left septal sub-endocardium. In panel (A), there is the recommended QRS polarity in leads II (slightly positive) and III (negative) while panel (B) illustrates the alternative, more inferior implantation site with negative QRS in both II and III. The nadir notch in V1 (‘W’ morphology) is observed in both cases. Notably, initial paced QRS morphologies anticipated the final portion of the left-sided conduction tissue that was engaged: in case (A), proximal LBB or LSF; in case (B), LPF.
A refinement of this method was proposed by dividing the septum into sectors. Liu et al.59 describe a technique using contrast injection in the RAO 30° view to identify the tricuspid annulus summit and to target a sector located 15–35 mm and between −10° and 30° from this point where LBB/fascicular potentials are often observed with LBBAP (see Figure 13).
Figure 13.
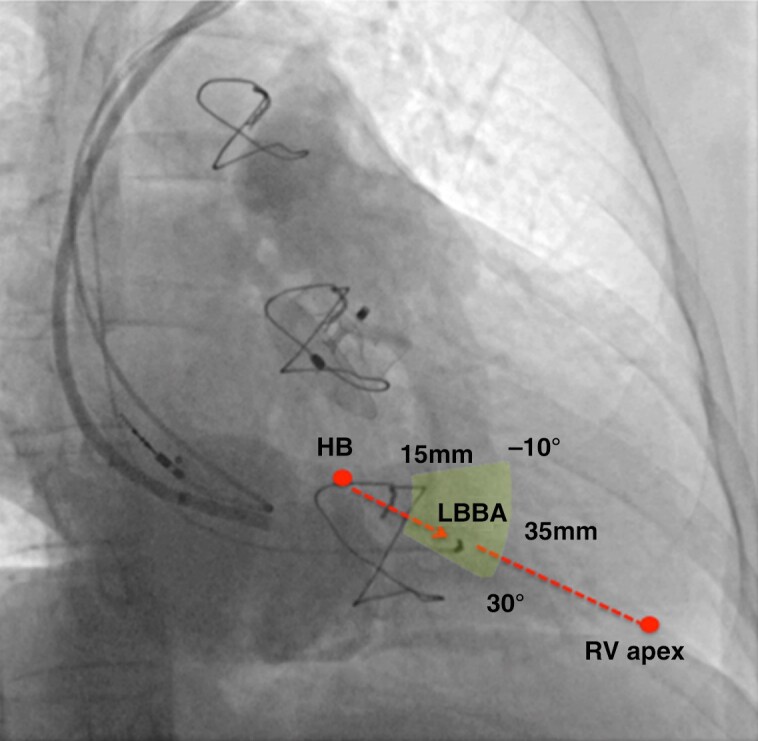
Insertion site for left bundle area pacing. In a RAO view at 30° with pacing lead in the left bundle area (LBBA) region, contrast is injected through a sheath delineating right atrial and ventricular anatomy. Tricuspid valve leaflets are identified by contrast. The summit of the tricuspid annulus indicates the approximate His bundle (HB) position. The red arrow indicates an imaginary line that connects the tricuspid annulus summit/His bundle with the RV apex, which can serve as a guide for placing the left bundle area lead. Successful pacing sites can be localized within a sector (indicated in yellow) located 15–35 mm away from the tricuspid annulus summit and at an angle of −10° to 30°, as described by Liu et al.59
The technique resulted in a significantly shorter procedure and fluoroscopic durations and a higher proportion of patients with visualization of fascicular potentials compared to their standard procedure. A ‘nine-partition method’ in which the RAO 30° fluoroscopic image of the RV is divided into 3 × 3 sectors has also been described.54,60 All these methods target sites which are further away from the His bundle region than the initially described target of 1.5–2 cm from the His bundle potential, suggesting that they correspond more to LFP than to LBBP. Placement of the LBBAP lead > 16 mm61 or >19 mm62 from the tricuspid annulus was associated with less tricuspid valve regurgitation than in a more proximal position.
If an atrial lead is also implanted, a modification of the above technique (‘dual lead technique’) can be used: one lead can be initially fixed at the His position serving as a reference during the implantation of the second lead in the LBB area. In patients with LBBB, the technique allows to identify a pre-systolic fascicular potential on the LBBAP lead if HBP corrects the conduction disorder.63 The first lead is later removed from the His bundle region and implanted in the atrium.
In patients with dilated right chambers, the delivery sheath may be reshaped with the dilator in situ to prevent kinking or inserted within a shortened coronary sinus catheter for extra support and reach.64 LBBAP lead implantation using electroanatomical mapping has been performed64,65 and may be useful in challenging anatomies.
Penetrating the interventricular septum
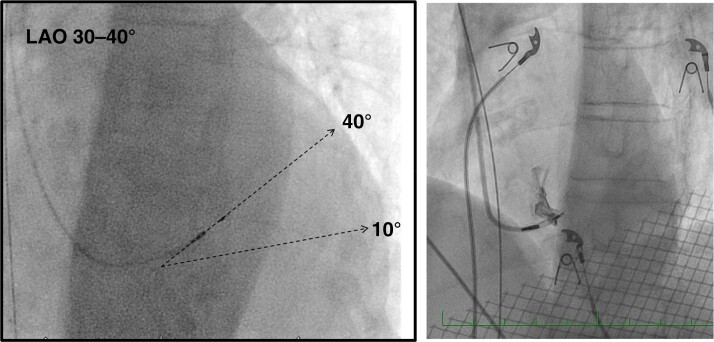
This is the key part of the LBBAP procedure. Once the targeted site for penetration of the interventricular septum is identified, the delivery catheter should be positioned perpendicular to the interventricular septum by a slight counter-clockwise rotation. A 30–40° left anterior oblique (LAO) view can confirm orientation of the lead. In this view, the lead should be oriented 10–40° (usually 20–30°) superiorly with respect to the horizontal plane (see Figure 14). Due to the curvature of the septum, a superior entry site may have a more horizontal orientation and an inferior site a more vertical orientation.
Figure 14.
Left panel: LAO view for orienting the lead 10–40° (most often 20–30°) with respect to the horizontal plane for perpendicular septal penetration. Right panel: example of lead orientation assisted by septography in the LAO view.
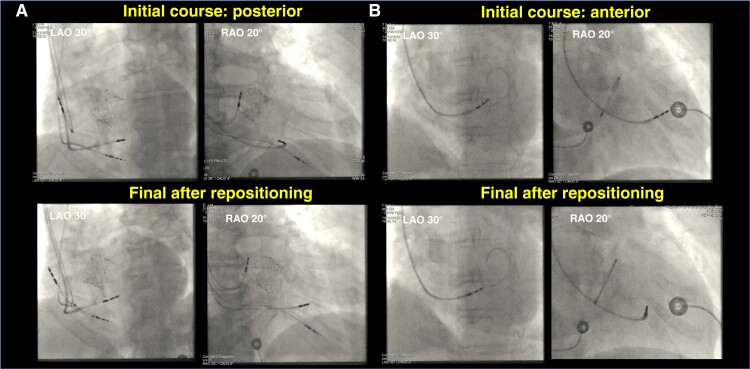
Checking lead orientation in RAO can be useful in case of difficulty with lead progression (due to a posterior course—see Figure 15A) or if no terminal r or R-wave appears in lead V1 and change in V6RWPT, despite apparent progression of the lead (due to an anterior course—see Figure 15B).
Figure 15.
Importance of the RAO view for evaluating issues with lead orientation which may appear adequate in the LAO view (top panels) and after re-positioning (bottom panels) in two patients. (A) Patient without clear progression of the lead in LAO view, with basal orientation of the lead revealed by the RAO view. (B) Another patient with apparent lead progression in the LAO view but without terminal R in V1 and no change in V6RWPT, with antero-apical orientation of the lead revealed by the RAO view.
The lead should be rapidly rotated with some forward push. Lumenless leads pull themselves into the septum with rotations of the body, whereas the larger SDLs may require more push.
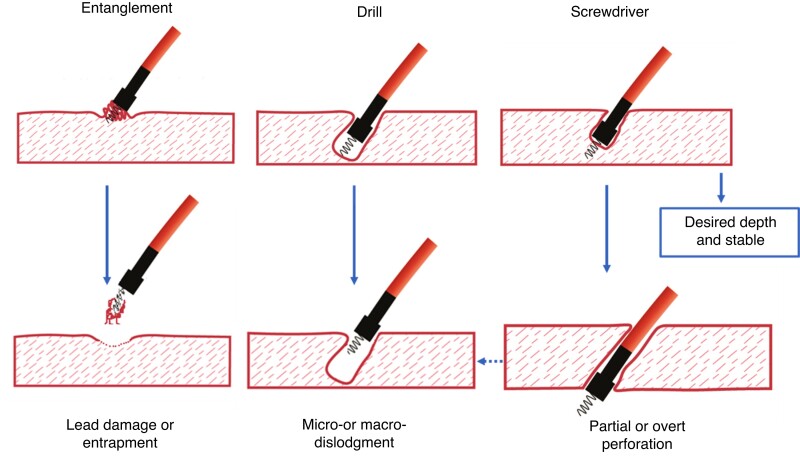
Assessment of lead behaviour during rotations facilitates proper lead deployment (see Figure 16). The ‘screwdriver effect’ is the desired smooth progression of the lead in the septum (see video S5).66 When strong torque build-up is observed after the initial rotations, ‘entanglement’ of the endocardium is usually present with little chance that the lead will penetrate. If the operator insists on trying to traverse the endocardium, it may be difficult to disentangle the lead for re-positioning without damaging the screw (see video S6). An undesirable ‘drill effect’ results from limited forward progression of the lead despite multiple rotations of the lead body (see video S7). In this instance, a tunnel is formed without proper securing of the lead by the screw in the tissue, which may result in lead dislodgment. A ‘drill’ effect should prompt either change of the position on the septum or increase of the lead push/support before further rotations.
Figure 16.
Lead behaviour during penetration of the septum during LBBAP. Both drill and screwdriver effects can result in perforation.
In case of difficulty with penetration of the septum, proper orientation of the delivery sheath and the lead should be checked, and the lead may be repositioned 1–2 cm in a superior, inferior, or apical direction. Push on the sheath may result in excessive bend of the proximal curve, with reduced torque transfer to the lead tip. Pulling back the sheath slightly after having anchored the lead with a few turns, while maintaining counter-clockwise torque to provide good contact and support, will straighten the proximal curve (ideally to <90°) and facilitate rotation of the lead and septal penetration. Contrast injection via the delivery catheter to delineate the curvature of the septum may optimize perpendicular orientation of the lead (see Figure 14). Change of sheath/lead with additional support can also be tried. As reported in the MELOS registry,7 penetration of the septum with the currently available tools may sometimes remain challenging, especially in heart failure patients, probably due to chamber enlargement, unfavourable septal orientation, and fibrosis.
Lead depth during rotations can be determined in various manners (frequently, more than one technique is needed to effectively estimate lead depth):
Continuous fluoroscopy in the LAO 30–40° view is useful, but progression of the lead is often quite subtle. It is impossible to determine appropriate lead depth with this method alone.
Unipolar paced QRS morphology. As the lead progresses from the right side to the left side of the septum, the QRS becomes narrower and loses notches/slurs, a Qr/qR/rsR’/R appears in V1, and the V6RWPT progressively shortens (Figure 17). When a terminal R-wave appears in V1, indicating RV activation delay, screwing of the lead should be interrupted to evaluate the LBBP ECG criteria described in detail below. Ideally, pacing should be un-interrupted during screwing of the lead, which is readily feasible with SDLs by connecting the crocodile clip cathode to the stylet.67 Revolving adapters are being developed for use with the 3830 lumenless lead.68,69
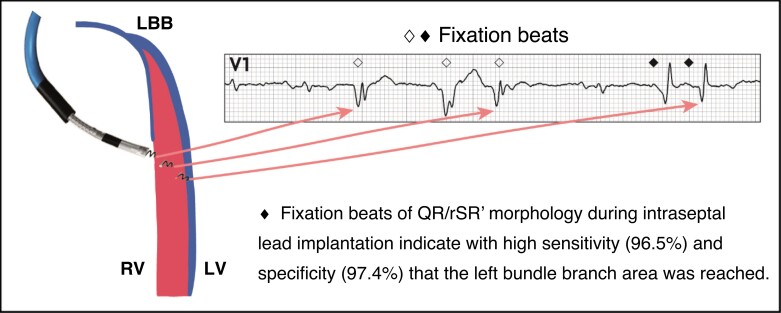
Fixation (template) beats. These are premature ventricular depolarizations that are evoked by the mechanical trauma as the lead passes through the interventricular septum and are observed in 59–96% of cases.70–72 Morphology of these beats corresponds very well with the actual depth of the lead tip and paced QRS complex from that location. Premature beats with terminal r or R-wave in V1 can serve as a marker that the lead rotations should be interrupted and ECG criteria tested since the lead is approaching or has reached the left subendocardial area.70,71 An example is shown in Figure 18. Fixation beats may be identical to selective LBB capture (with a rounded R’ in aVR and V1) and have been termed ‘M’ beats.69,72 Fixation beats which originate from conduction tissue may display an LBB/fascicular potential,52 which is useful in the setting of LBBP (where pre-systolic potentials are not visualized during conducted cycles).
Unipolar pacing impedance. This usually rises initially and then falls as the lead approaches the LV endocardium. Care should be taken when values approach 500 Ω or impedance drops by 200 Ω. It is important to note that pacing impedance depends not only on lead/tissue conduction but also upon the connections and cables and may vary from lab to lab. A steep rise in impedance with a standard lead indicates retraction of the extendible helix.
Myocardial COI. After an initial rise in amplitude (to about 20–35 mV), the sensed COI decreases as the lead reaches the LV subendocardial area to an average of approximately 10–12 mV (Figure 19).73,74 High COI gives the operator assurance that another lead rotation might be safely performed in the pursuit of LBB capture or better paced QRS. Therefore, if there is a clear drop-in COI amplitude (to approximately 5 mV based upon our experience), care with further screwing of the lead is advised (see section on complications). The COI may fall over 10 min and be accompanied by an improvement in capture thresholds if these were initially elevated.73 COI should be evaluated during unipolar sensing, but a fall in the COI during pacing may also be observed (Figure 19).75 The COI may fall/disappear with bipolar sensing configuration, but this has no pathological significance.
Visualization of an LBB/fascicular potential. These potentials are not visualized in all patients, but if present, they indicate that the subendocardial area has been reached and that any further rotations (e.g. to lower capture thresholds) should be performed with extreme caution as this may result in perforation. The presence of a COI of the potential indicates that further rotations are contraindicated.23 In the setting of LBBB, pre-systolic LBB/fascicular potentials are only visualized if LBBB is intermittent,76 in case of fixation beats originating from conduction tissue,52 or if a ‘dual lead technique’ is used with corrective HBP (see section 5.1). However, in some cases, a retrograde or delayed fascicular potential may be visualized within the ventricular electrogram (see Figure 20).
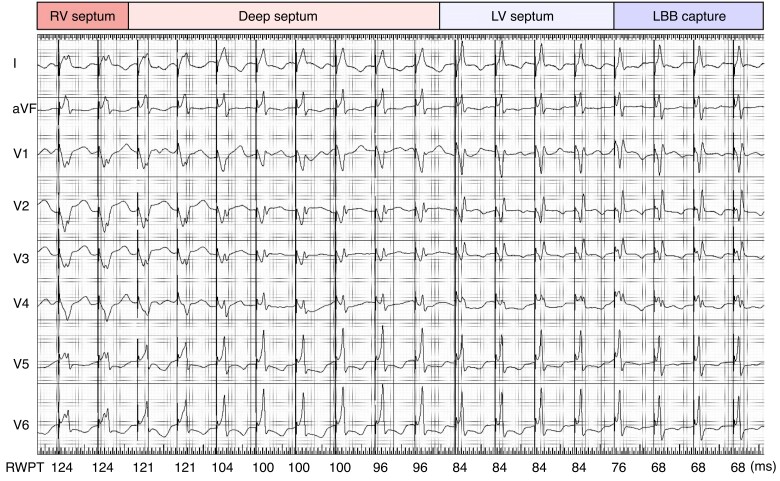
Figure 17.
Pacemapping for lead depth in the septum. Continuous pacing during intra-septal lead deployment enables to monitor continuous change of paced QRS complex morphology and lead depth in the septum. The right ventricular (RV septum) paced QRS is characterized by notches in lateral leads, ‘W’ morphology in V1, and time to R-wave peak (RWPT) in V6 > 120 ms. Deep septal paced QRS is narrower and loses notches in lateral leads, the notch in V1 moves towards the end of QRS, and V6RWPT is usually in the range of 120–95 ms. Pacing close to the left bundle branch area (LV septum) QRS is characterized by a positive terminal component in lead V1, pseudo-delta in leads V5–V6, and V6RWPT of 95–80 ms. LBB capture paced QRS is characterized by deeper S-wave in leads I, V5–V6, more prominent R in V1–V3, and V6RWPT usually <80 ms. LBB capture in the current case was assured both by V6RWPT < 74 ms and transition to selective capture (not shown).
Figure 18.
Fixation (or ‘template’) beats of different morphologies, reflecting depth of lead penetration. Reproduced with permission from Jastrzebski et al.70
Figure 19.
Myocardial COI during lead progression. A 15 s strip of the endocardial signals from the lead tip recorded during LBBAP implantation with continuous pacing at 100 bpm. Immediately after the premature fixation beat, preceded by a Purkinje potential (arrow), there is an obvious drop of the paced myocardial COI. Both COI drop and Purkinje potential are valuable markers indicating that the subendocardial area of the interventricular septum was reached by the pacing lead tip and that the lead rotations should be immediately stopped. In the present case, the lead rotations were continued and a further decrease in COI was observed (<4 mV and <25% of V wave), indicating imminent perforation. The endocardial signals (ENDO) from the pacing lead are acquired in unipolar mode and presented as filtered (30–100 Hz) and unfiltered (0.1–500 Hz). Sweep speed 12.5 mm/s. Reproduced, with permission from Jastrzebski et al.75
Figure 20.
Left bundle branch pacing lead implantation in a patient with LBBB, with electrograms during an intrinsic-conducted rhythm. A fascicular potential (arrows) is visualized within the ventricular electrogram during LBBB (first and last cycles) and is pre-systolic when the QRS narrows following a pause (after an atrial premature beat, not visible here). Modified, with permission, from Kaddour et al.77
An LBB/fascicular potential was visualized in 26.4% of patients in the MELOS registry,7 but in as many as 98.3% of cases in series targeting more proximal implantation sites.23
Contrast injection through the delivery catheter to delineate the septum can provide rough insight as to the depth of the lead (Figure 21).52 Simply advancing the delivery catheter up against the septum also gives an idea of lead depth.
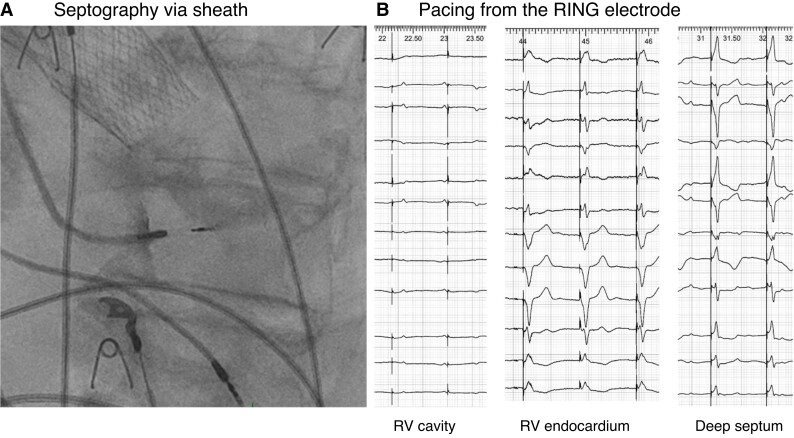
Capture with cathodal pacing from the ring electrode indicates that the lead has penetrated the septum to some extent (Figure 21). However, as with contrast injection, due to variable septal thickness and oblique course of the lead, it provides little information regarding proximity to the LBB area (despite knowing inter-electrode spacing).
‘Hinge point’ of the lead is an imprecise marker of lead depth, as it lies proximal to the ring electrode and will not give indication of how deep the lead tip is embedded. A rocking motion of the lead tip however indicates that there has been little penetration.
Figure 21.
(A) Contrast injection via delivery sheath to delineate the endocardial surface and depth of penetration of the intra-septal lead. (B) Pacing from the ring electrode also provides information regarding depth of penetration.
Confirmation of left conduction system capture and testing of electrical parameters
Differentiation between capture types during LBBAP is more challenging than for HBP and of less clear clinical significance. Even without direct LBB capture, indirect left conduction system engagement following myocardial capture can occur when the pacing lead is the subendocardial area of the LV septum. Visualization of an LBB or fascicular potential does not necessarily mean that conduction system capture will be obtained at low thresholds, and, conversely, conduction system capture at excellent thresholds may be obtained without the presence of a potential.
The most useful methods for confirming LBB capture are summarized below and covered in detail elsewhere.45,78,79
-
Transition in QRS morphology is the gold standard to assess capture. To demonstrate QRS transition, differences in excitability (threshold test) and/or refractoriness (programmed stimulation) between the conduction system and myocardium can be exploited.
Threshold test. Decremental voltage output pacing is the recommended initial method to confirm capture52 and should be performed in the unipolar mode to avoid transitions in QRS morphology due to anodal capture. The pacing amplitude is slowly decreased from high value (5–10 V@0.5 ms) to demonstrate a transition between simultaneous capture of LBB and septal myocardium (ns-LBBP) to selective capture of either only LBB (s-LBBP) or only septal myocardium (LVSP)—see Figure 22. If no transition is detected, then, the test is non-conclusive because capture thresholds of the LBB and septal myocardium may be nearly equal. This method has higher diagnostic yield when performed immediately after lead deployment, because the myocardial threshold is transiently elevated by acute injury. Only 26.4% of patients with LBB/fascicular capture showed this feature in the MELOS registry,7 but demonstration of s-LBBP has been reported in up to 75.4% of patients at implantation (and 30.9% at follow-up), targeting more proximal LBBP sites.63
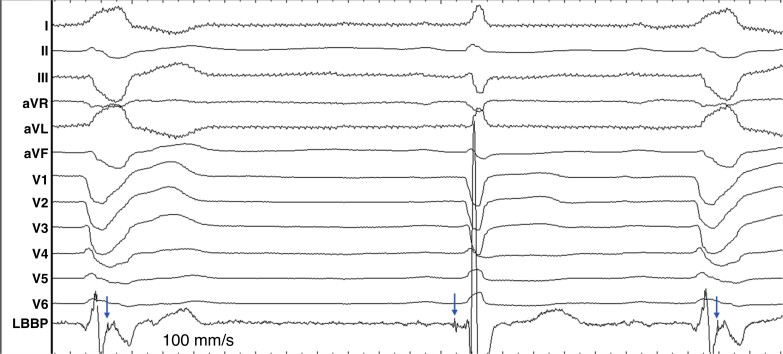
Physiology-based ECG criteria. With capture of the conduction system during LBBAP, the delay of the LBB potential to V6RWPT in intrinsic rhythm should equal that of the stimulus to V6RWPT during pacing (Figure 24). Allowing for variability in measurement, a difference <10 ms has a sensitivity of 88.2% and specificity of 95.4% for confirming conduction system capture.83 To increase the usefulness of this method for monitoring loss of LBB capture during follow-up, it is recommended that potential to V6 peak interval value be included in the implantation procedure report.
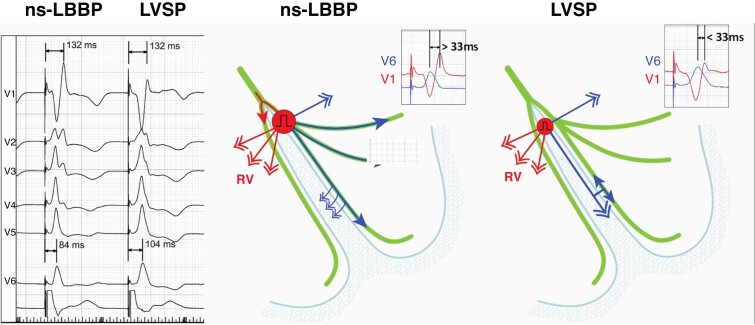
V6RWPT is often used as a surrogate of activation delay of the lateral LV. However, timing of local depolarization is more accurately evaluated by maximum dV/dT (which is impractical to use as it requires special software). During intrinsic rhythm with a narrow QRS, V6RWPT is <50 ms, while it is >60 ms in the presence of LBBB. During CSP, the same rules apply. The measurement is performed from the pacing stimulus to the peak of the R-wave in V6. Latency resulting from conduction tissue activation (approximately 30 ms, during which a pseudo-delta wave is visible due to myocardial capture) should be added to the 50 ms V6RWPT to confirm conduction tissue capture. In patients with narrow QRS or isolated RBBB, V6RWPT < 74 ms was 100% specific (albeit only 40% sensitive) for LBB capture, while a cut-off of ≤80 ms was 100% specific in patients with LBBB, NIVCD, and wide QRS escape rhythm.83 For practical purposes, cut-offs of <75 ms79 and <80 ms may be used respectively. Due to the variability of V6RWPT, with low sensitivity of the cut-offs, especially in patients with conduction disorders, additional methods are useful to confirm capture in case of prolonged V6RWPT. A caveat with V6RWPT is that this parameter was primarily studied in patients with a dominant R-wave in V5/6, and it is unclear if similar cut-offs can be used in case of an rS pattern in these leads.
Measurement of V6–V1 inter-peak interval. This last method is especially useful in patients with long V6RWPT due to substantial initial latency or global conduction slowing resulting in false-negative V6RWPT criterion. The V6–V1 interval criterion uses a patient-specific reference (V1 R-wave peak, reflecting RV activation) to assess LV activation and is less impacted by conduction system disease than V6RWPT (Figure 25). The optimal cut-off is >33 ms (which was validated in a separate report84), with a sensitivity of 71.8% and specificity of 90.0% for LBB capture, whereas > 44 ms was 100% specific.20 A caveat with this parameter is that it was primarily studied in patients with a dominant R-wave in V5/6, and it is unclear if similar cut-offs can be used in case of an rS pattern in these leads.
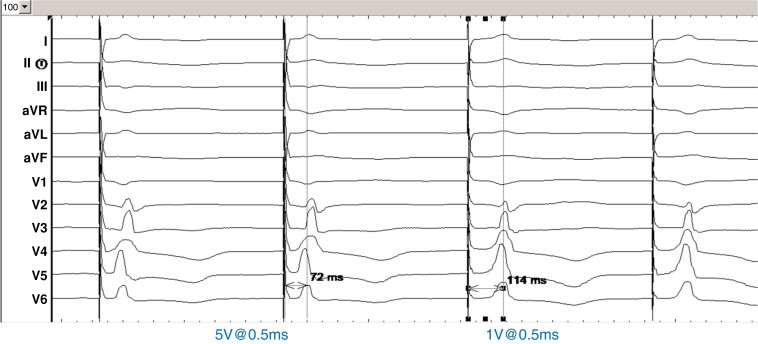
Sudden increase in V6RWPT ≥ 15 ms at reduced pacing output. This has a sensitivity of 82.6% and specificity of 100% for diagnosing loss of LBB capture.20 A value of >10 ms has also been proposed,79 but is within the range of inter-observer variability for measuring V6RWPT (which is about 12 ms20). An example is shown in Figure 26.
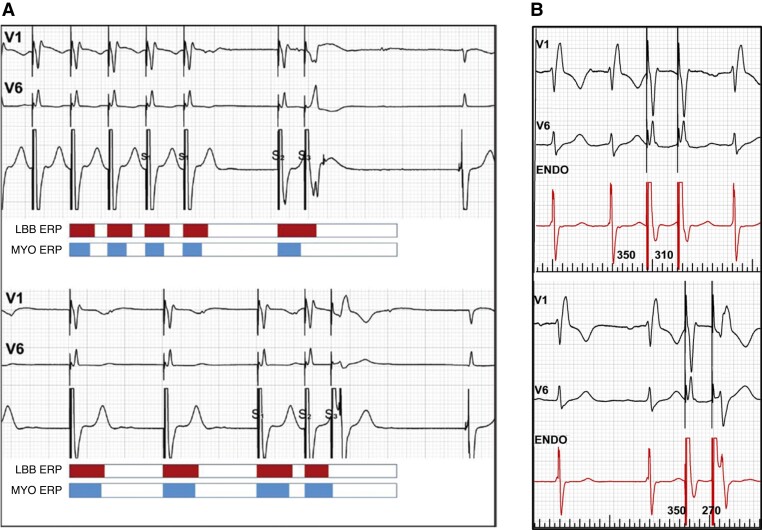
Programmed stimulation or burst/ramp pacing can be diagnostic when threshold testing is non-conclusive. To obtain selective response with S2 or S3, it is recommended to use pacing protocols that shorten refractoriness of the LBB and prolong refractoriness of the septal myocardium.80–82 This can be accomplished by a maximally long cycle of the drive (S1) and then two short-coupled extra-stimuli or alternatively two extra-stimuli delivered during intrinsic rhythm (Figure 23). A selective response is diagnostic of conduction system capture, whereas a myocardial response is less clear-cut due to changes in QRS morphology which may result from intra-myocardial conduction delay, without initial conduction system capture.
Figure 24.
Native and paced activation times of the left ventricle are equal when the conduction system is captured. LBB potential to V6RWPT is 80 ms, the same as stimulus to V6RWPT during ns-LBBP. In contrast, during loss of LBB capture, stimulus to V6RWPT is >10 ms longer than during intrinsic activation or ns-LBBP.
Figure 25.
Illustration of effect of ns-LBBP and loss of conduction tissue capture resulting in LVSP with myocardial capture only, on V6–V1 inter-peak interval, occurring during a threshold test. R-wave peak time in V1 reflects right ventricular activation that depends on transseptal conduction and is not affected by conduction tissue capture. RWPT in V6 reflects activation of the lateral wall of the left ventricle and is not influenced by loss of septal myocardial capture. Consequently, a longer V6–V1 interval is observed with left conduction system capture. Modified, with permission, from Jastrzebski et al.20
Figure 26.
Sudden prolongation of V6RWPT when reducing pacing output during LBBAP implantation, indicating loss of conduction system capture. An additional lead rotation resulted in conduction system capture threshold of 0.6V@0.5 ms. Note that other changes in QRS morphology are very subtle, and there is absence of a terminal r or R-wave in V1 despite the presence of conduction system capture.
Figure 23.
Programmed left bundle branch (LBB) area stimulation. (A) ‘Myocardial’ and selective LBB responses in the same patient. Top panel: fast drive shortens the refractoriness of septal myocardium; then, long pause (S2) prolongs refractoriness of the LBB; consequently, S3 finds myocardium excitable but LBB refractory (myocardial response). Bottom panel: slow drive prolongs refractoriness of the myocardium; then, short-coupled S2 shortens the refractoriness of the LBB; consequently, S3 finds myocardium refractory but LBB excitable (selective response). Reproduced with permission from Jastrzebski.82 (B) Double extra-stimuli delivered during intrinsic rhythm. Top panel: no change in QRS morphology with S2 coupling interval of 320 ms. Bottom panel: with S2 of 270 ms, a selective response is observed (note: also splitting of the signal in the endocardial channel).
An algorithm for confirming LBB/fascicular capture in clinical practice is shown in Figure 27.
Figure 27.
Algorithm for confirming conduction system capture with LBBAP. Some of the steps may be skipped according to operator preference, experience, or feasibility to perform particular measurements/manoeuvres. DSP = deep septal pacing; IVCD = intra-ventricular conduction delay; LBBAP = left bundle branch area pacing; LBBB = left bundle branch block; ns-LBBP = non-selective left bundle branch pacing; RBBB = right bundle branch block; RBBP = right bundle branch pacing; RWPT = R-wave peak time; s-LBBP = selective left bundle branch pacing.
It is important to understand that none of the proposed cut-offs are perfect—even parameters which are described as being 100% sensitive or specific in studies will have exceptions if the sample size is enlarged. Another aspect is that values vary according to individual patient profiles. Thus, a V6RWPT of 75 ms may represent LVSP in a female patient with a small heart and normal infra-nodal conduction, whereas a value of 100 ms in a large patient with dilated cardiomyopathy and bundle branch block may indicate conduction tissue capture.
Acceptable thresholds for LBBAP capture are <1.5 V@0.5 ms (ideally < 1 V@0.5 ms) and bipolar sensing should ideally be >4 mV. Capture thresholds may be initially high due to the COI and should be retested after a few minutes.
Anodal myocardial capture can lead to transitions in QRS morphology if threshold tests are performed with bipolar pacing (but should not be mistaken as proof of conduction system capture); it may in some instances be desirable as it may shorten the QRS duration.85 The capture threshold for anodal capture should be recorded if it is observed.
Slitting
As with HBP, before slitting the delivery catheter, any other additional leads should be implanted to avoid dislodging the LBBAP lead and to provide additional waiting time before retesting the lead. Lead stability may be tested before slitting, by gently pulling on the lead or by pushing and forming an α-shaped loop (see video S8). Paced QRS morphology and capture thresholds should be evaluated just before slitting. The sensed EGM should also be evaluated to check for the presence of a myocardial COI and LBB/fascicular potential (if observed initially) to evaluate perforation or micro-dislodgment.
The slitting tool should lock the lead in place and be maintained steadily while slitting the sheath. When using SDLs, the stylet should be partially removed (i.e. 10 cm) to avoid lead dislodgement while slitting.86,87
The electrical parameters should be checked again after slitting.
Considerations with stylet-driven pacing leads
SDLs differ from lumenless leads, in both lead and helix designs.86 Firstly, SDLs have an inner lumen for stylet insertion and therefore present with larger lead diameters (e.g. Abbott Tendril STS and BIOTRONIK Solia 5.6 F, Boston Scientific Ingevity+ 5.7 F, Microport Vega 6.0 F). Secondly, stylet insertion makes SDLs stiffer. Thirdly, most SDLs have an extendable-retractable helix design whereas lumenless leads have a fixed helix design. However, with standard leads, helix retraction can occur as the outer lead body turns over the inner coil and helix. This can be recognized by an increase in pacing impedance, as well as by observing the fluoroscopic markers. To avoid helix retraction during lead deployment, one should pretension the inner coil by additional rotations on the lead pin before locking it with the stylet insertion funnel (see video S9) or pinch tool (specific accessories are being developed for this purpose).
Pros and cons of SDLs vs. lumenless leads are summarized in Table 2.
Table 2.
Advantages and limitations of lumenless vs. stylet-driven leads for CSP
| Lumenless pacing leads | Stylet-driven pacing leads (SDLs) | |
|---|---|---|
| Potential advantages | Smaller lead body (4.1Fr) might result in less interference with septal kinetics
and less risk for collateral damage to septal vessels or septal
injury Isodiametric lead design facilitates septal penetration Fixed helix design: helix is robust and retraction will not occur In case of LBBP failure, HBP as bail out might be easier than with SDL |
Stylet-supported lead body resulting in enhanced stiffness and torquability,
facilitating deep septal lead deployment Delivery sheaths with larger diameters offer better support with less risk of kinking Wider lead body diameters (>5,5Fr) might offer more grip when rotating the outer lead body In case of lead dislodgment, standard right ventricular pacing may be performed without having to regain vascular access Extendible helix may be kept retracted during mapping and crossing the annulus, to avoid snagging tissue |
| Potential disadvantages | Absence of stylet results in less stiffness and torqu ability of the
lead Smaller lead body can result in less grip on the lead when rotating the lead Delivery sheaths with smaller diameters might be less supportive and more prone to kinking Requirement for new venous access and use of delivery sheath for re-positioning (even for conventional position) |
Extendable helix design might result in helix retraction during
screwing Higher risk of screw damage with re-positioning with current SDLs (especially for HBP) Larger lead diameter might increase the risk of collateral damage to septal vessel, septal injury, or interference with the tricuspid valve |
SDLs for His bundle pacing
According to a recent EHRA survey,88 SDLs are used only by a minority of physicians for HBP. The fixation mechanism of these leads may be relatively easily damaged when they are repositioned after having been fixated on the fibrous tissue of the His bundle region.89
Stylet-driven leads for left bundle area pacing
Most experience with LBBAP has been obtained with lumenless pacing leads.7,52,53,63,90 Recent reports have shown that LBBAP using standard SDLs is safe and feasible87,91–94 and are being used (exclusively or also with lumenless leads) by over half of implanting physicians according to the EHRA survey.88 Non-randomized single- and multi-centre studies report success rates of 87–95% for LBBAP with SDLs, with comparable pacing and electrophysiological characteristics to lumenless leads.87,92,93 As with lumenless leads, SDLs are positioned using pre-shaped delivery sheaths. The helix may be kept retracted during mapping of the His or the LBBAP lead insertion site (to avoid snagging) or, alternatively, extended. With the helix extended and the stylet fully inserted, clockwise rotation of the lead body allows to screw SDLs towards the left-sided septal area. While screwing, keeping the stylet advanced to the tip of the SDL enhances the stability and torquability, further facilitating deep septal lead deployment. Most often, stylets with medium stiffness suffice, although extra-firm stylets can be used if septal penetration is difficult. With SDL, unipolar pacing can be applied via the stylet, allowing continuous pacing and impedance monitoring while easily rotating the lead body.67,86
It is important to partially retract the stylet and sheath (and verify stability) before testing capture thresholds, to avoid confounding measurements due to support and extra lead tip contact offered by these tools.
The stiffer lead design of SDLs does not seem to result in more septal perforations compared to lumenless leads if adequate measures are taken to timely recognize impending perforation.87,92,93 Cases of SDL entanglement have been described, resulting in helix fracture, elongation, or disuse upon attempts to remove or reposition the lead.87,89,95 It is advisable to avoid entanglement by not continuing to rotate the lead if significant torque build-up is felt during lead fixation. If entanglement is suspected, counter-clockwise rotation and slight traction on the SDL while maintaining tension on the inner lead coil usually untangle the lead. Another concern with SDLs is a possibly higher rate of loss of LBB capture than with lumenless leads,96 which may have been due to the learning curve and which needs to be further evaluated.
Peri- and post-operative complications
The most frequent complication of HBP is a rise in capture threshold with loss of conduction system capture (in up to 17% of patients) or requiring lead revision (in up to 11% of patients).97 Sensing issues with ventricular undersensing or His/atrial oversensing may also occur.5
Complications of LBBAP and their incidences are summarized in Table 3.
Table 3.
Complications with LBBAP and their incidences
|
Per-operative complications
ȃSeptal perforation (0.0–14.1%)7,53,63,73,74,87,92,96,98–100 ȃRight bundle branch block (19.9% with 6.3% permanent)63 ȃComplete heart block (9.4% acute with 2.6% permanent)63 ȃIntra-operative lead dislodgment (3.0%)53 ȃAcute coronary syndrome (0.4–0.7%)7,101 ȃCoronary artery fistula (1.4–2.0%)87,92 ȃCoronary vein fistula/injury96,102 ȃSeptal hematoma103 ȃHelix damage/fracture (0.8–5.0%)87,89,95 |
|
Post-operative complications
ȃDelayed septal perforation (0.1–0.3%)7,87,104,105 ȃWorsening tricuspid regurgitation (7.3–32.6%)53,61–63 ȃLead dislodgment (0.3–1.5%)7,63,96,98,100,104,106,107 ȃRise in threshold by >1 V (0.3–1.8%)7,63,96,98,106 ȃLoss of LBB capture (0.3–11.5%)7,63,96 |
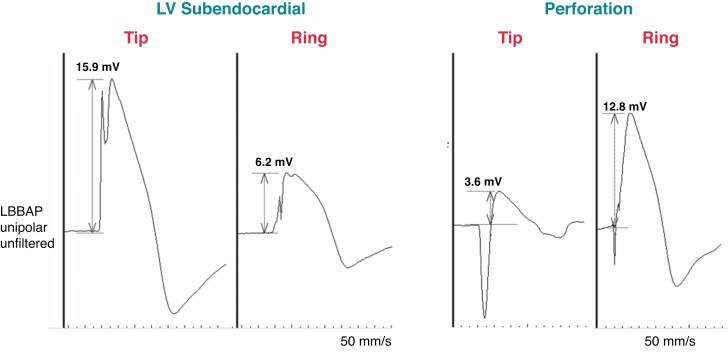
Perforation of the interventricular septum has been reported in 0–14.1% of patients. 7,53,63,74,92,98–100 Unipolar myocardial COI is an important indicator of perforation.23,73,74 Perforation is characterized by a COI ≤ 2.3 mV (mean 0.9 ± 0.6 mV) while good positions show COI of approximately 9 mV.24,73 Due to currently limited data, it is difficult at this stage to define an ideal cut-off that indicates perforation, but in our experience, when the unipolar tip COI is initially below 3–5 mV, perforation is to be suspected (the COI may however normally reduce in amplitude to these levels over the following 10 min and may also be significantly lower with bipolar sensing). Other useful markers are a COI < 25% of ventricular electrogram amplitude (sensitivity, specificity, and positive and negative predictive values of 100%, 94.62%, 28.95%, and 100%) and ring > tip COI amplitude (100%, 98.22%, 57.14%, and 100%, respectively).73 The morphology of the COI may also change with perforation, with a resulting QS or RS pattern (see Figure 28).24
Figure 28.
Current of injury morphology from the LBBAP lead in a patient with the lead in the left ventricular (LV) subendocardial region and in another patient with septal perforation. In contrast to adequate lead position, with perforation, the COI amplitude from the tip electrode is low and less than from the ring electrode, with a QR morphology indicating overt perforation (although unipolar capture was still possible here at 1.7 V/0.5 ms, with a pacing impedance of 380 Ω).
Other indicators of perforation are shown in Table 4 and include an acute fall in the pacing impedance < 450 Ω with the 3830 lead (sensitivity 100% and specificity 96.6%)24 or a fall in impedance by >200 Ω86 (although there are currently no published data on this parameter, this corresponds to the authors’ experience).
Table 4.
Indicators of septal perforation with LBBAP
| Myocardial COI amplitude |
| ȃCOI < 3–5 mV or absent |
| ȃCOI ring > tip73 |
| ȃCOI < 25% of V amplitude73 |
| Myocardial COI with QS or RS morphology74 |
| Drop-in unipolar pacing impedance to <450 Ω74 (or by >200 Ω) |
| Worsening of capture/sensing thresholds24,73 |
| Loss of LBB/fascicular potential23 |
| Contrast dye leakage into LV with injection via the delivery catheter87 |
| Overt perforation visualized by lead position/motion on fluoroscopy |
Overt perforation is usually straightforward to diagnose, either on fluoroscopy (see video S10) or due to loss of unipolar COI and capture. It can also be identified by leakage of contrast to the left ventricle with injection via the delivery catheter (see video S11). Micro-perforation (with the screw still partly in contact with excitable tissue) is however much more subtle to diagnose as capture/sensing thresholds may be preserved. Careful consideration of the morphological features described above, unipolar pacing impedance, and perioperative evolution of pacing thresholds are necessary to diagnose this complication.
If per-operative perforation is identified, it is important to reposition the lead (and not simply withdraw it slightly), as dislodgement due to the drill mechanism might occur. Acute perforation is asymptomatic and does not result in any known sequelae if the lead is repositioned. Late occurrence of septal perforation has been reported with an incidence of 0.1–0.3%.7,87,104,105 All cases of overt late perforation should be managed with lead re-positioning, and if delayed, oral anticoagulation should be initiated to prevent thromboembolism.
Micro-perforation may be diagnosed on post-operative cardiac imaging where a portion of the tip of the helix may be exposed to the LV cavity (see Figure 29). If electrical parameters remain stable with low capture thresholds, re-intervention is not warranted. Given the significant variability in the thickness of the septum, micro-perforation is likely an underappreciated phenomenon, and although a theoretical risk of thromboembolism is present, long-term anticoagulation is not indicated. To date, no reported strokes have been related to this complication. Most likely, prompt endothelialization reduces the thrombo-embolic risk.
Figure 29.
Micro-perforation of a 3830 lead in the LBB position with intact electrical parameters. No re-positioning was attempted, and there were no clinical sequelae.
The rate of lead macro-dislodgement is comparable to that of standard RV pacing. In LBBAP, lead tunnelling without forward progression into the septum (drill effect), inadequate slack, and neglecting to completely reposition the lead in the setting of acute perforation will increase the risk for dislodgement. Lead micro-dislodgement identified by loss of HBP/LB capture is probably underappreciated. Loss of terminal R-wave in V1 over follow-up was reported in 4% of patients in the MELOS registry.7 This highlights the importance of the 12-lead ECG at follow-up visits. Correct lead fixation, slitting technique, and adequate slack should reduce this likelihood. Requirement for lead re-positioning should be evaluated on a case-by-case basis.
Mechanical trauma to the septum, unavoidable during transseptal lead deployment, results in troponin level increase >3× over the normal range in 49.4% of patients. This was less than or comparable to other electrophysiology procedures,108 but higher compared to RV pacing lead implantation,109 although the rise may not be clinically meaningful. Septal myocardial injury may also occur during forceful contrast injection. For this reason, it is important that the sheath be pulled slightly off the septum during injection.
Mechanical trauma to coronary vessels is also possible during lead deployment. However, acute coronary events (due to direct damage to the septal perforators or coronary artery spasm) are very rarely observed. Myocardial infarction manifested with acute chest pain with ST segment elevation has only been reported in a few cases.7,101,104 Fistula with septal perforators or a coronary venous system and the right ventricle has been described but is rare and has not been reported to have clinical consequences.7
Worsening of tricuspid regurgitation is observed in up to a third of patients61 and is more frequent in patients with basal leads.61,62
Finally, the long-term effects of fatigue on the lead body at the hinge point proximal to the anode remains a concern.110 The current extraction experience of 3830 leads implanted in the LBB position is limited to case reports,110 whereas leads with longer dwell times will likely require special extraction tools.
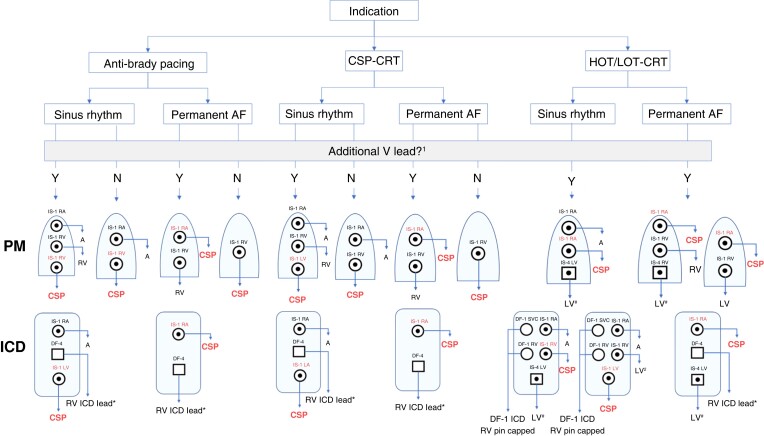
Configurations for device connection
As there are currently no generators dedicated to CSP, the choice of the device and the configuration for lead connection should be chosen according to the indication: (i) CSP instead of RV anti-bradycardia pacing, (ii) CSP instead of biventricular pacing, and (iii) CSP combined with ventricular pacing for optimizing CRT i.e. His-optimized CRT (HOT-CRT) or left bundle branch-optimized CRT (LOT-CRT). Further considerations are the requirement for an atrial lead (in case of sinus rhythm) and the requirement for additional ventricular lead(s) i.e. for ICD therapy, back-up ventricular pacing, adequate ventricular sensing, or hybrid pacing (HOT/LOT-CRT). Figure 30 shows the main combinations which are used. These different combinations require adapted programming, which is covered elsewhere.4,5
Figure 30.
Device configuration for CSP according to device indication, underlying rhythm (i.e. requirement for an atrial lead), presence on an additional ventricular lead. In all cases with a CSP lead plugged to the RV port, adequate sensing must be ensured. Not shown here are additional configurations with Y adapters and HOT-CRT with a His lead in combination with an RV lead only (which may be used in case of uncorrected selective His capture with isolated right bundle branch block111). 1Additional ventricular lead may be indicated for ICD therapy, back-up pacing, adequate ventricular sensing, or CRT optimization. 2The LV lead is plugged to the RV port in case the CSP lead does not provide proper sensing (atrial or His potential oversensing and ventricular undersensing are issues that may be encountered with HBP). *DF-1 ICD lead may also be used; #IS-1 LV lead may also be used. A = atrial; AF = atrial fibrillation; CRT = cardiac resynchronization therapy; CSP = conduction system pacing; HOT/LOT-CRT = His-optimized or left bundle pacing-optimized CRT; ICD = implantable cardioverter defibrillator; LV = left ventricle; PM = pacemaker; RA = right atrial; RV = right ventricle.
Follow-up
While many centres are increasingly utilizing remote pacemaker follow-up, the majority of CSP implanters prefer to include some in-house appointments. Most will perform an in-house check 4–8 weeks post-implantation and then a further in-person check at 6 months. The 2021 ESC pacing guidelines2 recommend in-person visits every 6 months with HBP due to the high incidence of late threshold rises and limitations with automatic capture threshold tests.112 Follow-up with LBBAP may be more spaced and also rely more on remote device management if capture thresholds are reliably measured by the device.
Paced 12-lead ECGs are mandatory during in-person follow-up to assess CSP capture and correction of bundle branch block. ECG belts can be useful for streamlining follow-up.6
Thresholds for conduction system capture, correction of bundle branch block, and myocardial thresholds should be reported. With LBBAP, it is worthwhile checking the QRS width in bipolar as well as unipolar configurations, as anodal capture may modify the QRS duration in some cases.85
Absence of atrial oversensing should be verified with HBP. Troubleshooting of CSP and optimization of programming are covered elsewhere.4,5
Future perspectives
CSP has been widely used worldwide using commercially available pacing leads and lead delivery systems, and indications are likely to broaden in the future.88,113,114 Nevertheless, many aspects of CSP lead implantation need to be improved. The pacing leads and delivery systems were not originally designed for CSP, and the implant tools are not suitable for challenging anatomies. Prototypes are in the pipeline, which will no doubt facilitate procedures. Leadless pacemakers designed for CSP are being developed.
PSAs which simultaneously display selected ECG leads (e.g. I, II, III, V1, and V6) and EGMs (also unfiltered), with the possibility of performing measurements with digital callipers, would facilitate CSP implantations in centres without an EP recording system.
Generators designed for the purposes of CSP would facilitate programming and follow-up and may offer new options such as the ability to deliver dual cathodal pacing (tip and ring electrodes) in order that the left bundle and the right ventricle may be captured with fused activation and better synchrony. Artificial intelligence may serve to identify conduction system capture and facilitate testing at implantation and follow-up.
Long-term lead performance and the safety of extraction of the LBBAP lead need to be carefully studied. Specific extraction tools may need to be developed. More data from large, randomized clinical trials with long-term follow-up are required before CSP can be considered as the optimal pacing therapy and be firmly incorporated in guidelines.
Finally, although LBBAP is overtaking HBP,88 the latter procedure has advantages which justify its continued use (see Table 5).
Table 5.
Advantages and limitations of HBP and of LBBAP
| HBP | LBBAP |
|---|---|
| Advantages | Advantages |
| Maximum electrical synchrony Endpoints well-defined for successful His capture Extractability has been demonstrated Relatively good mid-term evidence for safety and efficacy Avoids crossing the tricuspid valve when implanted on the atrial aspect of the annulus) Some evidence of medium and long-term lead extraction115,116 |
Large target area Correction of more distal conduction disease Low capture thresholds Good sensing parameters Consistent back-up myocardial capture (in addition by anodal capture by the ring electrode) No requirement for back-up pacing leads AV nodal ablation without risk of compromising lead function |
| Disadvantages/limitations | Disadvantages/limitations |
| Small target area Capture thresholds may be high Sensing issues (atrial and His oversensing, ventricular undersensing) Limited to correction of proximal conduction block only Risk if distal conduction block develops over follow-up High (up to 11%97) requirement for lead revision Back-up ventricular leads may be indicated in specific situations Complex programming in case of back-up leads Risk of compromising lead function with AV node ablation32,33,117 |
Conduction tissue capture may be difficult to demonstrate in some
cases Requirement of digital callipers (i.e. electrophysiology recording system) for measuring parameters of conduction system capture Less electrical synchrony compared to HBP, especially in patients with normal baseline QRS Complications specific to transseptal route (septal perforation, lesions to coronary vessels, septal hematoma, etc.) Tricuspid regurgitation53,62,63 May be challenging in patients with septal scar Limited (but growing) evidence for safety and efficacy Long-term extractability needs to be demonstrated |
Conclusions
CSP has emerged as being one of the most exciting developments in pacing therapy over the last years and is progressively gaining mainstream clinical practice worldwide. Although limited, the currently available data heralds a promising future for this therapy, which should nevertheless be implemented in a safe and effective manner.
New tools are very likely to facilitate the procedure, as has been the case with coronary sinus lead implantation in the past. Nevertheless, operator skill will remain a requirement to deliver therapy safely and effectively. Standardization of the procedure and proper education are aspects which will help operators gain that skill to deliver more physiological pacing therapy to their patients. The purpose of the present document is to facilitate that process.
| STRENGTH OF ADVICE | Symbol |
|---|---|
| Clinical advice, based on robust published evidence |
|
| Clinical advice, based on consensus of the writing group |
|
| May be appropriate, based on published evidence |
|
| May be appropriate, based on consensus of the writing group |
|
| Areas of uncertainty |
|
| STRENGTH OF EVIDENCE | Abbreviation |
|---|---|
| Randomized controlled trials | RCT |
| Meta-analysis | META |
| Observational studies | OBS |
| Expert opinion | OPN |
| GENERAL CONSIDERATIONS | Evidence | Strength |
|---|---|---|
| Advice TO DO | ||
| 12-lead ECG should be recorded during conduction system pacing implantation, ideally using an electrophysiology recording system for simultaneous endocardial and ECG signals. | OPN |

|
| Endocavitary electrograms with minimally filtered signals showing current of injury should be displayed.24,73 | OPN |

|
| May be appropriate TO DO | ||
| If an EP recording system is not available, use of a 12-lead ECG with a pacing system analyzer for mapping endocardial signals may be used.7 | OBS |

|
| Advice NOT TO DO | ||
| CSP implantation should not be performed without a minimum display of ECG leads I, II, III, V1 and V5 or V6. | OPN |

|
| HIS BUNDLE PACING | Evidence | Strength |
|---|---|---|
| Advice TO DO | ||
| His bundle capture should always be confirmed at implantation and follow-up, using validated criteria46–49 such as transitions in QRS morphology with decrementing output / programmed stimulation and comparison of intervals from His potential and from pacing stimulus to V6 R-wave peak. | OBS |

|
| Screwing of the lead should be continued until significant torque buildup is felt to ensure lead stability | OPN |

|
| Lead stability should be routinely assessed43 | OBS |

|
| May be appropriate TO DO | ||
| In case of infranodal conduction delay or block, pacing at 400 ms or shorter cycle length showing 1:1 conduction without aberration should be tested.50 | OBS |

|
| Lead rotations should be pursued until a His current of injury or deep negative morphology is observed, which predicts good electrical parameters.22,42 | OBS |

|
| Unipolar His capture thresholds should be <2.5 V / 0.5 ms but should ideally aim for ≤1.5 V / 0.5 ms. Bipolar sensing amplitude should be >2 mV (without atrial/His oversensing) | OPN |

|
| In the interest of safety, a backup ventricular lead can be useful in specific situations such as poor sensing, pacemaker-dependency, high-grade AVB, infranodal block, high pacing threshold and planned AVJ ablation. | OPN |

|
| Areas of uncertainty | ||
| Both selective as well as non-selective HBP are acceptable and probably have similar outcome although non-selective HBP provides the safety of ventricular backup capture and higher sensing amplitudes.16–19 | OBS |

|
| LEFT BUNDLE BRANCH AREA PACING | Evidence | Strength |
|---|---|---|
| Advice TO DO | ||
| Lead depth should be monitored using different techniques such as fluoroscopy, unipolar paced QRS morphology and impedance, fixation/template beat morphology,71,72 presence of a fascicular potential,23 current of injury amplitude,24,73 contrast injection via the delivery catheter and capture by the ring electrode. | OBS |

|
| It is appropriate to evaluate and report presence or absence of conduction system capture using validated criteria20,80,83 such as transition in QRS morphology with decrementing output/programmed stimulation, V6RWPT, V6-V1 interpeak interval, comparison of intervals from intrinsic potential and from pacing stimulus to V6 R-wave peak. | OBS |

|
| Lead entanglement at the insertion site (characterized by torque build-up) or a ‘drill’ effect during screwing (recognized by lack of lead progression on fluoroscopy and by pace-mapping despite lead rotations) should be recognized to avoid lead damage and dislodgement respectively.66 | OBS |

|
| Acceptable thresholds for LBBAP capture are <1.5 V @ 0.5 ms (ideally < 1 V @ 0.5 ms) and bipolar sensing should ideally be >4 mV. | OPN |

|
| May be appropriate TO DO | ||
| Lead insertion site is evaluated based upon fluoroscopy in the 20–30° right anterior oblique view at 15–35 mm from the His bundle / tricuspid annulus and using pacemapping.59 | OBS |

|
| The lead can be screwed into the interventricular septum at an angle of 10–40° superior to the horizontal plane in the left anterior oblique 30–40° view. | OBS |

|
| Advice NOT TO DO | ||
| In case of perforation of the septum, the lead should be repositioned at a new site and not simply withdrawn slightly. | OPN |

|
| Areas of uncertainty | ||
| It is unclear at this point if conduction system capture with left bundle branch area pacing is necessary to achieve good clinical outcome.13,118–122 | OBS |

|
Tables of advice
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
The authors thank the EHRA scientific documents committee: Prof. Katja Zeppenfeld, Prof. Jens Cosedis Nielsen, Dr. Alireza Sepehri Shamloo, Prof. Kristina Hermann Haugaa, Prof. Isabel Deisenhofer, Prof. Silvia Priori, Prof. Markus Stühlinger, Prof. Christian Meyer, Prof. Petr Peichel, Dr. Daniel Keene, Prof. Jacob Tfelt Hansen, Dr. Luigi di Biase, and Prof. Arthur Wilde.
Contributor Information
Haran Burri, Cardiac Pacing Unit, Cardiology Department, University Hospital of Geneva, Rue Gabrielle Perret Gentil 4, 1211 Geneva 14, Switzerland.
Marek Jastrzebski, First Department of Cardiology and Electrocardiology and Arterial Hypertension, Jagiellonian University, Kopernika 17, 31-501 Kraków, Poland.
Óscar Cano, Hospital Universitario y Politecnico La Fe, Avinguda de Fernando Abril Martorell 106, 46026 Valencia, Spain; Centro de Investigaciones Biomédicas en RED en Enfermedades Cardiovasculares (CIBERCV), Av. Monforte de Lemos, 3-5 Pabellón 11, Planta 0 28029 Madrid, Spain.
Karol Čurila, Cardiocenter, Third Faculty of Medicine, Charles University and University Hospital, Kralovske Vinohrady, Ruská 87, 100 00 Prague 10, Czech Republic.
Jan de Pooter, Heart Centre, University Hospital Ghent, Corneel Heymanslaan 10, 9000 Gent, Belgium.
Weijian Huang, The First Affiliated Hospital of Wenzhou Medical University, Nanbaixiang, Wenzhou 325000, China.
Carsten Israel, Bethel-Clinic, Burgsteig 13, 33617, Bielefeld, Germany.
Jacqueline Joza, Department of Medicine, McGill University Health Center, 1001 Decarie Boulevard, H4A 3J1 Montreal (Quebec), Canada.
Jorge Romero, Brigham and Women's Hospital, 75 Francis St, Boston, MA 02115, USA.
Kevin Vernooy, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Universiteitssingel 50, 6229 Maastricht, the Netherlands.
Pugazhendhi Vijayaraman, Geisinger Wyoming Valley Medical Center, 1000 East Mountain Blvd., Wilkes-Barre, PA 18711, USA.
Zachary Whinnett, National Heart and Lung Institute, Imperial College London, Guy Scadding Building, Dovehouse St, London SW3 6LY, UK.
Francesco Zanon, Arrhythmia and Electrophysiology Unit, Santa Maria della Misericordia General Hospital, Viale Tre Martiri 140, 45100 Rovigo, Italy.
Document reviewers
Jean-Claude Deharo (EHRA review coordinator) (France), Bernard Thibault (Canada), Frédéric Anselme (France), Wei Hua (China), Michael Glikson (Israel), Francisco Moscoso Costa (Portugal), Jens Cosedis Nielsen (Denmark), Daniel Keene (United Kingdom), Markus Stühlinger (Austria), and Emmanuel Simantirakis (Grece).
Disclosure
H.B. has received speaker honoraria, consulting fees, or institutional fellowship/research support from Abbot, BIOTRONIK, Boston Scientific, Medtronic, and MicroPort. C.I. has received speaker honoraria or consulting fees from Abbot, BIOTRONIK, Boston Scientific, Medtronic and MicroPort, as well as research support from MicroPort. O.C. has received speaker honoraria and consulting fees from BIOTRONIK, Boston Scientific, and Medtronic, K.C. has received speaker honoraria and consulting fees from BIOTRONIK, Boston Scientific, and Medtronic. J.D.P. reports speaker honoraria and consultancy fees from Medtronic, BIOTRONIK, Abbott, and Boston Scientific. J.J. reports support from Medtronic (investigator-initiated external research grant) and Boston Scientific (consulting). KV has received speaker honoraria, consulting fees, or institutional fellowship/research support from Abbot, Boston Scientific, Medtronic, and MicroPort. P.V. has the following declarations: Medtronic—speaker, consultant, research, and fellowship support; Abbott—consultant; BIOTRONIK and Boston Scientific—honoraria; Patent—HBP delivery tool. Z.W. has received speaker honoraria, consulting fees, or institutional fellowship/research support from Abbot, Boston Scientific, and Medtronic. The other authors have not reported any disclosures.
References
- 1. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom-Lundqvist Cet al. . 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. The task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 2. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. . 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 3. Burri H, Starck C. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS)-a role for postoperative ultrasound? Authors’ reply. Europace 2021;24:523–24. [DOI] [PubMed] [Google Scholar]
- 4. Bakelants E, Burri H. Troubleshooting programming of conduction system pacing. Arrhythm Electrophysiol Rev 2021;10:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burri H, Keene D, Whinnett Z, Zanon F, Vijayaraman P. Device programming for His bundle pacing. Circ Arrhythm Electrophysiol 2019;12:e006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakelants E, Zweerink A, Burri H. Programming and follow-up of patients with His bundle pacing. Herzschrittmacherther Elektrophysiol 2020;31:177–82. [DOI] [PubMed] [Google Scholar]
- 7. Jastrzebski M, Kielbasa G, Cano O, Curila K, Heckman L, De Pooter Jet al. . Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burri H, Jastrzebski M, Vijayaraman P. Electrocardiographic analysis for His bundle pacing at implantation and follow-up. JACC Clin Electrophysiol 2020;6:883–900. [DOI] [PubMed] [Google Scholar]
- 9. Vijayaraman P. Deep septal, distal His bundle pacing for cardiac resynchronization therapy. HeartRhythm Case Rep 2020;6:791–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jastrzebski M, Kielbasa G, Moskal P, Bednarek A, Rajzer M, Curila Ket al. . Proximal right bundle branch pacing: criteria, characteristics and outcomes. Heart Rhythm 2023; S1547-5271(23)00099-1. doi:10.1016/j.hrthm.2023.01.017. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 11. Burri H. Fortuitous right bundle branch pacing. Heart Rhythm Case Reports 2023;9:61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cabrera J-Á, Porta-Sánchez A, Tung R, Sánchez-Quintana D. Tracking down the anatomy of the left bundle branch to optimize left bundle branch pacing. JACC Case Rep 2020;2:750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu S, Sharma PS, Huang W. Novel left ventricular cardiac synchronization: left ventricular septal pacing or left bundle branch pacing? Europace 2020;22:ii10–8. [DOI] [PubMed] [Google Scholar]
- 14. Williams DO, Scherlag BJ, Hope RR, El-Sherif N, Lazzara R, Samet P. Selective versus non-selective His bundle pacing. Cardiovasc Res 1976;10:91–100. [DOI] [PubMed] [Google Scholar]
- 15. Saini A, Serafini NJ, Campbell S, Waugh WB, Zimberg R, Sheldon TJet al. . Novel method for assessment of His bundle pacing morphology using near field and far field device electrograms. Circ Arrhythm Electrophysiol 2019;12:e006878. [DOI] [PubMed] [Google Scholar]
- 16. Bednarek A, Ionita O, Moskal P, Linkova H, Kiełbasa G, Prochazkova Ret al. . Nonselective versus selective His bundle pacing: an acute intrapatient speckle-tracking strain echocardiographic study. J Cardiovasc Electrophysiol 2021;32:117–25. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Guo J, Hou X, Wang Y, Qian Z, Li Ket al. . Comparison of the effects of selective and non-selective His bundle pacing on cardiac electrical and mechanical synchrony. Europace 2018;20:1010–7. [DOI] [PubMed] [Google Scholar]
- 18. Curila K, Prochazkova R, Jurak P, Jastrzebski M, Halamek J, Moskal Pet al. . Both selective and nonselective His bundle, but not myocardial, pacing preserve ventricular electrical synchrony assessed by ultra-high-frequency ECG. Heart Rhythm 2020;17:607–14. [DOI] [PubMed] [Google Scholar]
- 19. Beer D, Sharma PS, Subzposh FA, Naperkowski A, Pietrasik GM, Durr Bet al. . Clinical outcomes of selective versus nonselective His bundle pacing. JACC Clin Electrophysiol 2019;5:766–74. [DOI] [PubMed] [Google Scholar]
- 20. Jastrzębski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek Aet al. . The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace 2022;24:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Leon J, Seow SC, Boey E, Soh R, Tan E, Gan HHet al. . Adopting permanent His bundle pacing: learning curves and medium-term outcomes. Europace 2022;24:606–13. [DOI] [PubMed] [Google Scholar]
- 22. Vijayaraman P, Dandamudi G, Worsnick S, Ellenbogen KA. Acute His-bundle injury current during permanent His-bundle pacing predicts excellent pacing outcomes. Pacing Clin Electrophysiol 2015;38:540–6. [DOI] [PubMed] [Google Scholar]
- 23. Su L, Xu T, Cai M, Xu L, Vijayaraman P, Sharma PSet al. . Electrophysiological characteristics and clinical values of left bundle branch current of injury in left bundle branch pacing. J Cardiovasc Electrophysiol 2020;31:834–42. [DOI] [PubMed] [Google Scholar]
- 24. Ponnusamy SS, Basil W, Vijayaraman P. Electrophysiological characteristics of septal perforation during left bundle branch pacing. Heart Rhythm 2022;19:728–34. [DOI] [PubMed] [Google Scholar]
- 25. Vijayaraman P, Dandamudi G. How to perform permanent His bundle pacing: tips and tricks. Pacing Clin Electrophysiol 2016;39:1298–304. [DOI] [PubMed] [Google Scholar]
- 26. Padala SK, Cabrera JA, Ellenbogen KA. Anatomy of the cardiac conduction system. Pacing Clin Electrophysiol 2021;44:15–25. [DOI] [PubMed] [Google Scholar]
- 27. Sato T, Soejima K, Maeda A, Mohri T, Katsume Y, Tashiro Met al. . Safety of distal His bundle pacing via the right ventricle backed up by adjacent ventricular capture. JACC Clin Electrophysiol 2021;7:513–21. [DOI] [PubMed] [Google Scholar]
- 28. Vijayaraman P, Dandamudi G, Subzposh FA, Shepard RK, Kalahasty G, Padala SKet al. . Imaging-Based localization of His bundle pacing electrodes: results from the prospective IMAGE-HBP study. JACC Clin Electrophysiol 2021;7:73–84. [DOI] [PubMed] [Google Scholar]
- 29. Hu Y, Gu M, Hua W, Niu H, Li H, Chen Xet al. . Electrical characteristics of pacing different portions of the His bundle in bradycardia patients. Europace 2020;22:ii27–35. [DOI] [PubMed] [Google Scholar]
- 30. Vijayaraman P, Dandamudi G. Anatomical approach to permanent His bundle pacing: optimizing His bundle capture. J Electrocardiol 2016;49:649–57. [DOI] [PubMed] [Google Scholar]
- 31. Vijayaraman P, Herweg B, Dandamudi G, Mittal S, Bhatt AG, Marcantoni Let al. . Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: insights into disease progression. Heart Rhythm 2019;16:1554–61. [DOI] [PubMed] [Google Scholar]
- 32. Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace 2017;19:iv10–6. [DOI] [PubMed] [Google Scholar]
- 33. Zweerink A, Bakelants E, Stettler C, Burri H. Cryoablation vs. radiofrequency ablation of the atrioventricular node in patients with His-bundle pacing. Europace 2021;23:421–30. [DOI] [PubMed] [Google Scholar]
- 34. Acosta H, Acosta N, Viafara G, de Las Salas A, Pothula SR, Acosta NMet al. . A novel and practical technique to facilitate lead insertion at the His bundle region. J Interv Card Electrophysiol 2021. doi:10.1007/s10840-021-00941-z. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 35. Gu M, Niu H, Hu Y, Liu X, Zhang N, Cai Met al. . Permanent His bundle pacing implantation facilitated by visualization of the tricuspid valve annulus. Circ Arrhythm Electrophysiol 2020;13:e008370. [DOI] [PubMed] [Google Scholar]
- 36. Sharma PS, Huang HD, Trohman RG, Naperkowski A, Ellenbogen KA, Vijayaraman P. Low fluoroscopy permanent His bundle pacing using electroanatomic mapping: a feasibility study. Circ Arrhythm Electrophysiol 2019;12:e006967. [DOI] [PubMed] [Google Scholar]
- 37. Orlov MV, Koulouridis I, Monin AJ, Casavant D, Maslov M, Erez Aet al. . Direct visualization of the His bundle pacing lead placement by 3-dimensional electroanatomic mapping: technique, anatomy, and practical considerations. Circ Arrhythm Electrophysiol 2019;12:e006801. [DOI] [PubMed] [Google Scholar]
- 38. Vijayaraman P, Mascarenhas V. Three-dimensional mapping-guided permanent His bundle pacing in a patient with corrected transposition of great arteries. HeartRhythm Case Rep 2019;5:600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imnadze G, Vijayaraman P, Bante H, Eitz T, Bergau L, Baridwan Net al. . Novel electroanatomical map for permanent His bundle pacing: the Mont Blanc approach - influence of the learning curve and procedural outcome. Europace 2020;22:1697–702. [DOI] [PubMed] [Google Scholar]
- 40. Namboodiri N, Kakarla S, Mohanan Nair KK, Abhilash SP, Saravanan S, Pandey HKet al. . Three-dimensional electroanatomical mapping guided right bundle branch pacing in congenitally corrected transposition of great arteries. Europace 2022: euac239. doi:10.1093/europace/euac239. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zanon F, Abdelrahman M, Marcantoni L, Naperkowski A, Subzposh FA, Pastore Get al. . Long term performance and safety of His bundle pacing: a multicenter experience. J Cardiovasc Electrophysiol 2019;30:1594–601. [DOI] [PubMed] [Google Scholar]
- 42. Sato T, Soejima K, Maeda A, Mohri T, Tashiro M, Momose Yet al. . Deep negative deflection in unipolar His-bundle electrogram as a predictor of excellent His-bundle pacing threshold postimplant. Circ Arrhythm Electrophysiol 2019;12:e007415. [DOI] [PubMed] [Google Scholar]
- 43. Su L, Wu S, Wang S, Wang Z, Xiao F, Shan Pet al. . Pacing parameters and success rates of permanent His-bundle pacing in patients with narrow QRS: a single-centre experience. Europace 2019;21:763–70. [DOI] [PubMed] [Google Scholar]
- 44. Jastrzębski M. Physiologic differentiation between selective His bundle, nonselective His bundle and septal pacing. Card Electrophysiol Clin 2022;14:151–63. [DOI] [PubMed] [Google Scholar]
- 45. Jastrzebski M. ECG and pacing criteria for differentiating conduction system pacing from myocardial pacing. Arrhythm Electrophysiol Rev 2021;10:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jastrzebski M, Moskal P, Curila K, Fijorek K, Kukla P, Bednarek Aet al. . Electrocardiographic characterization of non-selective His-bundle pacing: validation of novel diagnostic criteria. Europace 2019;21:1857–64. [DOI] [PubMed] [Google Scholar]
- 47. Jastrzębski M, Moskal P, Kukla P, Bednarek A, Kiełbasa G, Rajzer Met al. . Novel approach to diagnosis of His bundle capture using individualized left ventricular lateral wall activation time as reference. J Cardiovasc Electrophysiol 2021;32:3010–8. [DOI] [PubMed] [Google Scholar]
- 48. Jastrzebski M, Moskal P, Bednarek A, Kielbasa G, Vijayaraman P, Czarnecka D. His bundle has a shorter chronaxie than does the adjacent ventricular myocardium: implications for pacemaker programming. Heart Rhythm 2019;16:1808–16. [DOI] [PubMed] [Google Scholar]
- 49. Jastrzebski M, Moskal P, Bednarek A, Kielbasa G, Vijayaraman P, Czarnecka D. Programmed His bundle pacing. Circ Arrhythm Electrophysiol 2019;12:e007052. [DOI] [PubMed] [Google Scholar]
- 50. Katritsis DG, Josephson ME. Electrophysiological testing for the investigation of bradycardias. Arrhythm Electrophysiol Rev 2017;6:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beer D, Subzposh FA, Colburn S, Naperkowski A, Vijayaraman P. His bundle pacing capture threshold stability during long-term follow-up and correlation with lead slack. Europace 2021;23:757–66. [DOI] [PubMed] [Google Scholar]
- 52. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019;16:1791–6. [DOI] [PubMed] [Google Scholar]
- 53. Vijayaraman P, Subzposh FA, Naperkowski A, Panikkath R, John K, Mascarenhas Vet al. . Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019;16:1774–82. [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Wang Z, Zu L, Cheng L, Su R, Wang Xet al. . Simplifying physiological left bundle branch area pacing using a new nine-partition method. Can J Cardiol 2021;37:329–38. [DOI] [PubMed] [Google Scholar]
- 55. Chen K, Li Y. How to implant left bundle branch pacing lead in routine clinical practice. J Cardiovasc Electrophysiol 2019;30:2569–77. [DOI] [PubMed] [Google Scholar]
- 56. Padala SK, Master VM, Terricabras M, Chiocchini A, Garg A, Kron Jet al. . Initial experience, safety, and feasibility of left bundle branch area pacing: a multicenter prospective study. JACC: Clinical Electrophysiology 2020;6:1773–82. [DOI] [PubMed] [Google Scholar]
- 57. Zhang S, Zhou X, Gold MR. Left bundle branch pacing: JACC review topic of the week. J Am Coll Cardiol 2019;74:3039–49. [DOI] [PubMed] [Google Scholar]
- 58. Ponnusamy SS, Arora V, Namboodiri N, Kumar V, Kapoor A, Vijayaraman P. Left bundle branch pacing: a comprehensive review. J Cardiovasc Electrophysiol 2020;31:2462–73. [DOI] [PubMed] [Google Scholar]
- 59. Liu X, Niu HX, Gu M, Chen X, Hu Y, Cai Met al. . Contrast-enhanced image-guided lead deployment for left bundle branch pacing. Heart Rhythm 2021;18:1318–25. [DOI] [PubMed] [Google Scholar]
- 60. Jiang H, Hou X, Qian Z, Wang Y, Tang L, Qiu Yet al. . A novel 9-partition method using fluoroscopic images for guiding left bundle branch pacing. Heart Rhythm 2020;17:1759–67. [DOI] [PubMed] [Google Scholar]
- 61. Hu Q, You H, Chen K, Dai Y, Lu W, Li Yet al. . Distance between the lead-implanted site and tricuspid valve annulus in patients with left bundle branch pacing: effects on postoperative tricuspid regurgitation deterioration. Heart Rhythm 2023;20:217–23. [DOI] [PubMed] [Google Scholar]
- 62. Li X, Zhu H, Fan X, Wang Q, Wang Z, Li Het al. . Tricuspid regurgitation outcomes in left bundle branch area pacing and comparison with right ventricular septal pacing. Heart Rhythm 2022;19:1202–3. [DOI] [PubMed] [Google Scholar]
- 63. Su L, Wang S, Wu S, Xu L, Huang Z, Chen Xet al. . Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circulation Arrhythmia and electrophysiology 2021;14:e009261. [DOI] [PubMed] [Google Scholar]
- 64. O'Connor M, Riad O, Shi R, Hunnybun D, Li W, Jarman JWEet al. . Left bundle branch area pacing in congenital heart disease. Europace 2022;25:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Richter S, Gebauer R, Ebert M, Moscoso Ludueña C, Scheller D, Lucas Jet al. . Electroanatomical mapping-guided left bundle branch area pacing in patients with structural heart disease and advanced conduction abnormalities. Europace 2023;25:1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jastrzebski M, Moskal P, Holda MK, Strona M, Bednarek A, Kielbasa Get al. . Deep septal deployment of a thin, lumenless pacing lead: a translational cadaver simulation study. Europace 2020;22:156–61. [DOI] [PubMed] [Google Scholar]
- 67. Gillis K, O'Neill L, Wielandts JY, Hilfiker G, Vlase A, Knecht Set al. . Left bundle branch area pacing guided by continuous uninterrupted monitoring of unipolar pacing characteristics. J Cardiovasc Electrophysiol 2022;33:299–307. [DOI] [PubMed] [Google Scholar]
- 68. Shen J, Jiang L, Cai X, Wu H, Pan L. Left bundle branch pacing guided by continuous pacing technique that can monitor electrocardiograms and electrograms in real time: a technical report. Can J Cardiol 2022;38:1315–7. [DOI] [PubMed] [Google Scholar]
- 69. Jastrzębski M, Moskal P. Reaching the left bundle branch pacing area within 36 heartbeats. Kardiol Pol 2021;79:587–8. [DOI] [PubMed] [Google Scholar]
- 70. Jastrzebski M, Kielbasa G, Moskal P, Bednarek A, Kusiak A, Sondej Tet al. . Fixation beats: a novel marker for reaching the left bundle branch area during deep septal lead implantation. Heart Rhythm 2021;18:562–9. [DOI] [PubMed] [Google Scholar]
- 71. Ponnusamy SS, Ganesan V, Syed T, Balasubramanian S, Vijayaraman P. Template beat: a novel marker for left bundle branch capture during physiological pacing. Circ Arrhythm Electrophysiol 2021;14:e009677. [DOI] [PubMed] [Google Scholar]
- 72. Ponnusamy SS, Basil W, Vijayaraman P. M-beat-a novel marker for selective left bundle branch capture. J Cardiovasc Electrophysiol 2022;33:1888–92. [DOI] [PubMed] [Google Scholar]
- 73. Shali S, Wu W, Bai J, Wang W, Qin S, Wang Jet al. . Current of injury is an indicator of lead depth and performance during left bundle branch pacing lead implantation. Heart Rhythm 2022;19:1281–8. [DOI] [PubMed] [Google Scholar]
- 74. Ponnusamy S, Basil W, Vijayaraman P. Electrophysiological characteristics of septal perforation during left bundle branch pacing. Heart Rhythm 2022;19:728–34. [DOI] [PubMed] [Google Scholar]
- 75. Jastrzębski M. Left bundle branch area pacing lead implantation using an uninterrupted monitoring of endocardial signals. J Cardiovasc Electrophysiol 2022;33:1055–7. [DOI] [PubMed] [Google Scholar]
- 76. Vijayaraman P, Huang W. Atrioventricular block at the distal His bundle: electrophysiological insights from left bundle branch pacing. HeartRhythm Case Rep 2019;5:233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaddour M, Burri H. Case report of hidden (yet visible) systolic fascicular potentials in a patient with left bundle branch block during conduction system pacing implantation. Eur Heart J Case Rep 2023;7:ytad024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ponnusamy SS, Vijayaraman P. Evaluation of criteria for left bundle branch capture. Card Electrophysiol Clin 2022;14:191–202. [DOI] [PubMed] [Google Scholar]
- 79. Wu S, Chen X, Wang S, Xu L, Xiao F, Huang Zet al. . Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. JACC Clin Electrophysiol 2021;7:1166–77. [DOI] [PubMed] [Google Scholar]
- 80. Jastrzębski M, Moskal P, Bednarek A, Kiełbasa G, Kusiak A, Sondej Tet al. . Programmed deep septal stimulation: a novel maneuver for the diagnosis of left bundle branch capture during permanent pacing. J Cardiovasc Electrophysiol 2020;31:485–93. [DOI] [PubMed] [Google Scholar]
- 81. Denker S, Lehmann MH, Mahmud R, Gilbert C, Akhtar M. Divergence between refractoriness of His-Purkinje system and ventricular muscle with abrupt changes in cycle length. Circulation 1983;68:1212–21. [DOI] [PubMed] [Google Scholar]
- 82. Jastrzębski M. Permanent left bundle branch pacing: what is the mechanism of divergent responses during programmed stimulation? J Cardiovasc Electrophysiol 2020;31:1222–5. [DOI] [PubMed] [Google Scholar]
- 83. Jastrzębski M, Kiełbasa G, Curila K, Moskal P, Bednarek A, Rajzer Met al. . Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm 2021;18:935–43. [DOI] [PubMed] [Google Scholar]
- 84. Briongos-Figuero S, Estévez-Paniagua Á, Sánchez-Hernández A, Muñoz-Aguilera R. Combination of current and new electrocardiographic-based criteria: a novel score for the discrimination of left bundle branch capture. Europace 2023;25:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu H, Jiang L, Shen J, Zhang L, Zhong J, Zhuo S. The electrophysiological characteristics and possible mechanism of bipolar pacing in left bundle branch pacing. Heart Rhythm 2022;19:2019–26. [DOI] [PubMed] [Google Scholar]
- 86. De Pooter J, Wauters A, Van Heuverswyn F, Le Polain de Waroux JB. A guide to left bundle branch area pacing using stylet-driven pacing leads. Front Cardiovasc Med 2022;9:844152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. De Pooter J, Ozpak E, Calle S, Peytchev P, Heggermont W, Marchandise Set al. . Initial experience of left bundle branch area pacing using stylet-driven pacing leads: a multicenter study. J Cardiovasc Electrophysiol 2022;33:1540–9. [DOI] [PubMed] [Google Scholar]
- 88. Kircanski B, Boveda S, Prinzen F, Sorgente A, Anic A, Conte Get al. . Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace 2023;25:682–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tan ESJ, Lee JY, Boey E, Soh R, Sim MG, Yeo WTet al. . Use of extendable helix leads for conduction system pacing: differences in lead handling and performance lead design impacts conduction system pacing. J Cardiovasc Electrophysiol 2022;33:1550–7. [DOI] [PubMed] [Google Scholar]
- 90. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou Xet al. . A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–e3. [DOI] [PubMed] [Google Scholar]
- 91. Byeon K, Kim HR, Park SJ, Park YJ, Choi JH, Kim JYet al. . Initial experience with left bundle branch area pacing with conventional stylet-driven extendable screw-in leads and new pre-shaped delivery sheaths. J Clin Med 2022;11:2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. De Pooter J, Calle S, Timmermans F, Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol 2021;32:439–48. [DOI] [PubMed] [Google Scholar]
- 93.Jastrzębski M. Multicenter European Left Bundle Branch Area Pacing Outcomes Study (MELOS). Eur Heart J 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zanon F, Marcantoni L, Pastore G, Baracca E. Left bundle branch pacing by standard stylet-driven lead: preliminary experience of two case reports. HeartRhythm Case Rep 2020;6:614–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Le Polain de Waroux JB, Wielandts JY, Gillis K, Hilfiker G, Sorgente A, Capulzini Let al. . Repositioning and extraction of stylet-driven pacing leads with extendable helix used for left bundle branch area pacing. J Cardiovasc Electrophysiol 2021;32:1464–6. [DOI] [PubMed] [Google Scholar]
- 96. Tan ESJ, Lee JY, Boey E, Soh R, Seow SC, Teo LJTet al. . Predictors of loss of capture in left bundle branch pacing: a multicenter experience. Heart Rhythm 2022;19:1757–58. [DOI] [PubMed] [Google Scholar]
- 97. Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron Jet al. . Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm 2021;18:743–9. [DOI] [PubMed] [Google Scholar]
- 98. Padala SK, Master VM, Terricabras M, Chiocchini A, Garg A, Kron Jet al. . Initial experience, safety, and feasibility of left bundle branch area pacing: a multicenter prospective study. JACC Clin Electrophysiol 2020;6:1773–82. [DOI] [PubMed] [Google Scholar]
- 99. Hua W, Fan X, Li X, Niu H, Gu M, Ning Xet al. . Comparison of left bundle branch and His bundle pacing in bradycardia patients. JACC Clin Electrophysiol 2020;6:1291–9. [DOI] [PubMed] [Google Scholar]
- 100. Vijayaraman P, Ponnusamy S, Cano Ó, Sharma PS, Naperkowski A, Subsposh FAet al. . Left bundle branch area pacing for cardiac resynchronization therapy: results from the international LBBAP collaborative study group. JACC Clin Electrophysiol 2021;7:135–47. [DOI] [PubMed] [Google Scholar]
- 101. Ponnusamy SS, Vijayaraman P. Aborted ST-elevation myocardial infarction-an unusual complication of left bundle branch pacing. HeartRhythm Case Rep 2020;6:520–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Molina-Lerma M, Tercedor-Sánchez L, Molina-Jiménez M, Álvarez M. Visualization of a septal perforator branch vein and coronary sinus during left bundle pacing implant. Eur Heart J Case Rep 2021;5:ytab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zheng R, Wu S, Wang S, Su L, Ellenbogen KA, Huang W. Case report: interventricular septal hematoma complicating left bundle branch pacing lead implantation. Front Cardiovasc Med 2021;8:744079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen X, Wei L, Bai J, Wang W, Qin S, Wang Jet al. . Procedure-related complications of left bundle branch pacing: a single-center experience. Front Cardiovasc Med 2021;8:645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ravi V, Larsen T, Ooms S, Trohman R, Sharma PS. Late-onset interventricular septal perforation from left bundle branch pacing. HeartRhythm Case Rep 2020;6:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sharma PS, Patel NR, Ravi V, Zalavadia DV, Dommaraju S, Garg Vet al. . Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush conduction system pacing registry. Heart Rhythm 2022;19:3–11. [DOI] [PubMed] [Google Scholar]
- 107. Ponnusamy SS, Vijayaraman P. Late dislodgement of left bundle branch pacing lead and successful extraction. J Cardiovasc Electrophysiol 2021;32:2346–9. [DOI] [PubMed] [Google Scholar]
- 108. Ponnusamy SS, Patel NR, Naperkowski A, Subzposh FA, Vijayaraman P. Cardiac troponin release following left bundle branch pacing. J Cardiovasc Electrophysiol 2021;32:851–5. [DOI] [PubMed] [Google Scholar]
- 109. Chen X, Jin Q, Bai J, Wang W, Qin S, Wang Jet al. . The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace 2020;22:ii36–44. [DOI] [PubMed] [Google Scholar]
- 110. Burri H. Complications with left bundle branch area pacing. Heart Rhythm 2022;19:735–6. [DOI] [PubMed] [Google Scholar]
- 111. Ellenbogen KA, Padala SK. Taking the heat out of cardiac resynchronization therapy and conduction system pacing. JACC: Clinical Electrophysiology 2021;7:893–5. [DOI] [PubMed] [Google Scholar]
- 112. Starr N, Dayal N, Domenichini G, Stettler C, Burri H. Electrical parameters with His-bundle pacing: considerations for automated programming. Heart Rhythm 2019;16:1817–24. [DOI] [PubMed] [Google Scholar]
- 113. Kaza N, Htun V, Miyazawa A, Simader F, Porter B, Howard JPet al. . Upgrading right ventricular pacemakers to biventricular pacing or conduction system pacing: a systematic review and meta-analysis. Europace 2022;euac188. doi:10.1093/europace/euac188. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Badertscher P, Knecht S, Zeljković I, Sticherling C, de Asmundis C, Conte Get al. . Management of conduction disorders after transcatheter aortic valve implantation: results of the EHRA survey. Europace 2022;24:1179–85. [DOI] [PubMed] [Google Scholar]
- 115. Vijayaraman P, Subzposh FA, Naperkowski A. Extraction of the permanent His bundle pacing lead: safety outcomes and feasibility of reimplantation. Heart Rhythm 2019;16:1196–203. [DOI] [PubMed] [Google Scholar]
- 116. Migliore F, Dall'Aglio P, Falzone PV, Bertaglia E, Zanon F. Extraction of a very old His bundle pacing lead: a safe and effective procedure? Pacing Clin Electrophysiol 2021;44:1464–5. [DOI] [PubMed] [Google Scholar]
- 117. Su L, Cai M, Wu S, Wang S, Xu T, Vijayaraman Pet al. . Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace 2020;22:ii19–26. [DOI] [PubMed] [Google Scholar]
- 118. Curila K, Jurak P, Jastrzebski M, Prinzen F, Waldauf P, Halamek Jet al. . Left bundle branch pacing compared to left ventricular septal myocardial pacing increases interventricular dyssynchrony but accelerates left ventricular lateral wall depolarization. Heart Rhythm 2021;18:1281–9. [DOI] [PubMed] [Google Scholar]
- 119. Curila K, Jurak P, Vernooy K, Jastrzebski M, Waldauf P, Prinzen Fet al. . Left ventricular myocardial septal pacing in close proximity to LBB does not prolong the duration of the left ventricular lateral wall depolarization compared to LBB pacing. Front Cardiovasc Med 2021;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Heckman LIB, Luermans J, Curila K, Van Stipdonk AMW, Westra S, Smisek Ret al. . Comparing ventricular synchrony in left bundle branch and left ventricular septal pacing in pacemaker patients. J Clin Med 2021;10:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mirolo A, Chaumont C, Auquier N, Savoure A, Godin B, Vandevelde Fet al. . Left bundle branch area pacing in patients with baseline narrow, left, or right bundle branch block QRS patterns: insights into electrocardiographic and echocardiographic features. Europace 2023;25:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hou X, Qian Z, Wang Y, Qiu Y, Chen X, Jiang Het al. . Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. Europace 2019;21:1694–702. [DOI] [PubMed] [Google Scholar]