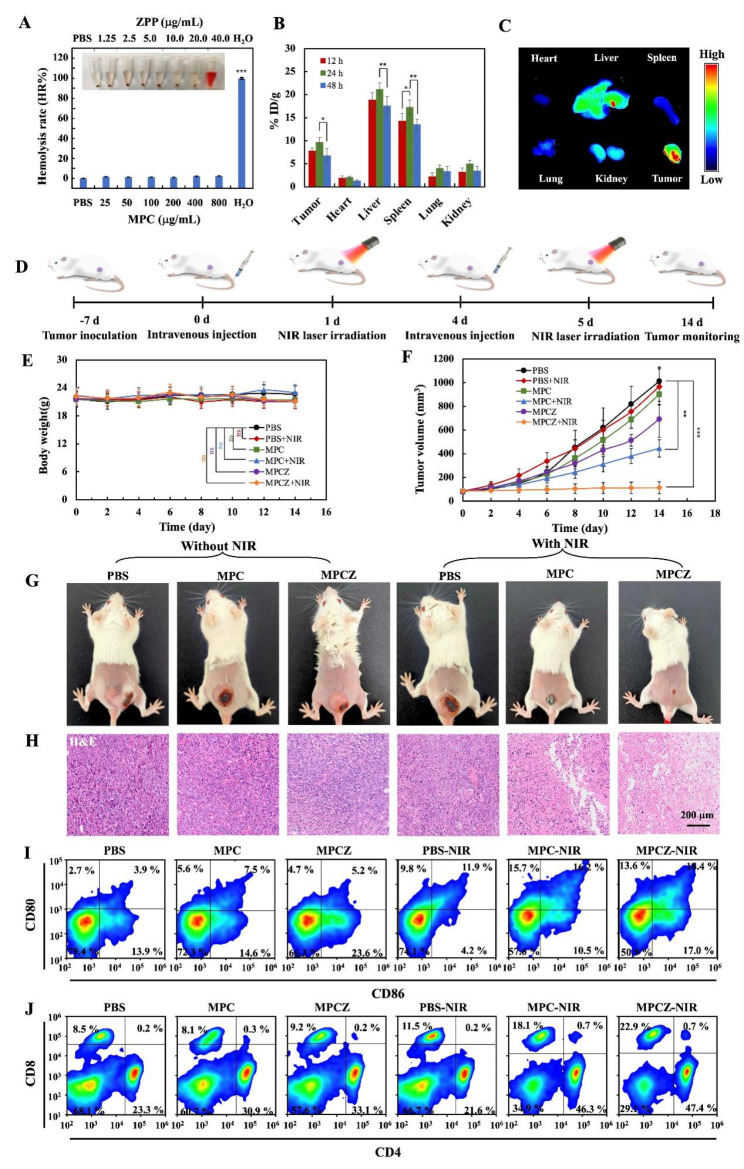
Fig. 6.
In vivo the anti-tumor and immunotherapy effects of MPCZ NPs. (A) Digital photograph of hemolysis test and hemolysis rate (HR%) of MPCZ NPs. (B) ICP-MS analysis of Mn elements in tissues dissected from mice at 12, 24 or 48 h post-administration with MPCZ NPs (data expressed as percentage of the injected dose per gram of tissue (% ID/g)). (C) Fluorescence images of main organs and tumors at 24 h post-injection of MPCZ NPs. (D) Schematic illustration of MPCZ NPs therapy. (E) Weight change of mice during the treatment period. (F) Tumor growth curves of PBS, MPC NPs and MPCZ NPs (comparable to 20 mg/kg mouse for MPC and 1 mg/kg mouse for ZPP) treated 4T1 tumor-bearing mice with or without 808 nm laser irradiation. (G) Images of tumor-bearing mice after 14 days of different treatments. (H) Tumor tissue staining by H&E after 14 days of treatments. (I) Frequency of CD80+ CD86+ DCs in tumor-draining lymph nodes (gate on CD11c+ DCs). (J) Frequency of CD4+ and CD8+ DCs in tumor-draining lymph nodes (gate on CD3+ T cells). For (A), (B), (E) and (F), the asterisks represent statistical significance in comparison to the PBS group based on one-way ANOVA with Tukey post-hoc analysis. *p < 0.05, ** p < 0.01, ***p < 0.001