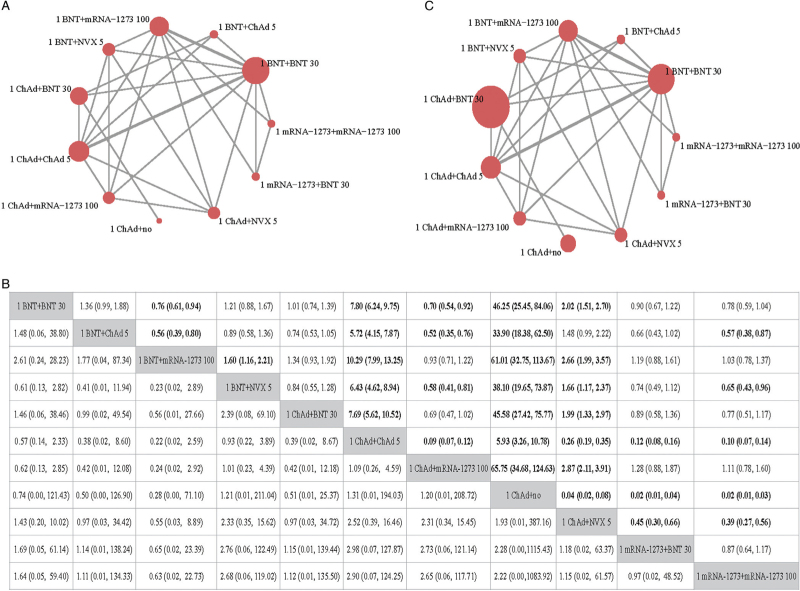
Figure 3.
Results from NMA of primary outcomes of schedules initiated with mRNA or non-replicating viral vector. (A) Evidence network of the neutralizing antibody against an original strain. (B) Evidence network of SAEs. The figure in each cell in the upper area refers to the OR of the schedule in the row against the schedule in the column, and the figure in each cell in the lower area refers to the OR of the schedule in the column against the schedule in the row. If no relevant information on SAEs or neutralizing antibody is reported in a comparison, the a dot will be used to fill the cell in the corresponding position. (C) GMR and 95% CI of comparisons (the results for SAEs are presented in the lower area, and the results for neutralizing antibodies are presented in the upper area). 1 ChAd + NVX 5: one dose of ChAdOx1 followed by NVX-CoV2373 5 μg; 1 mRNA-1273 + BNT 30: one dose of mRNA-1273 followed by BNT162b2 30 μg; 1 mRNA-1273 + mRNA-1273 100: one dose of mRNA-1273 followed by mRNA-1273 100 μg; 1 BNT + BNT 30: one dose of BNT162b2 followed by BNT162b1 30 μg; 1 BNT + ChAd 5: one dose of BNT162b2 followed by ChAdOx1 5 × 1010 viral particles; 1 BNT + mRNA-1273 100: one dose of BNT162b2 followed by mRNA-1273 100 μg; 1 BNT + NVX 5: one dose of BNT162b2 followed by NVX-CoV2373 5 μg; 1 ChAd + BNT 30: one dose of ChAdOx1 followed by BNT162b2 30 μg; 1 ChAd + ChAd 5: one dose of ChAdOx1 followed by ChAdOx1 5 × 1010 viral particles; 1 ChAd + mRNA-1273 100: one dose of ChAdOx1 followed by mRNA-1273 100 μg; 1 ChAd + no: one dose of ChAdOx1 only. CI: Confidence interval; GMR: Geometric mean ratio; NMA: Network meta-analysis; SAEs: Serious adverse events.