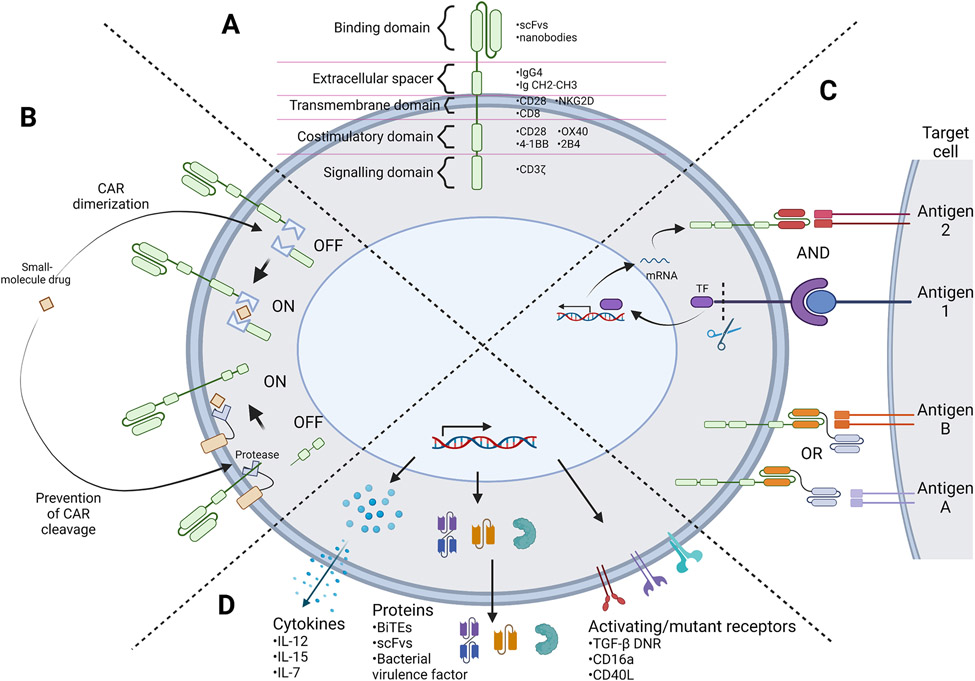
Figure 1.
Representation of synthetic biology strategies for engineering next-generation T and NK cell therapies. (A) Schematic depicting the CAR protein architecture along with commonly employed domains in each modular component. (B) Example of CAR regulation strategies. A small-molecule drug can act as a dimerizing agent to assemble a full CAR protein, or stabilize CAR protein expression by inhibiting a protease to prevent CAR protein cleavage. (C) AND and OR Boolean logic-gate strategies for multi-antigen sensing. AND-gated strategies require the sensing of multiple tumor antigens to trigger target cell killing. For example, recognition of antigen 1 can prompt the release of a transcription factor (TF) that triggers expression of a CAR specific to antigen 2, thus both antigens 1 and 2 must be present in order to trigger CAR-T cell activation. OR-gated strategies require the sensing of any one antigen among multiple antigens to trigger target cell killing. For example, tandem bispecific OR-gate CARs have two binding domains that recognize two different antigens, such that recognition of either antigen A or B will induce activation of the CAR-expressing cell. (D) Armoring strategies in which transgenic payloads are expressed together with the CAR transgene. These payloads can be cytokines, chemokines, receptors, or other proteins that aim to improve the function of the T or NK cell and/or neighboring immune cells, frequently with the goal of reprogramming the surrounding tumor microenvironment. (Figure created with BioRender.)