Figure 1.
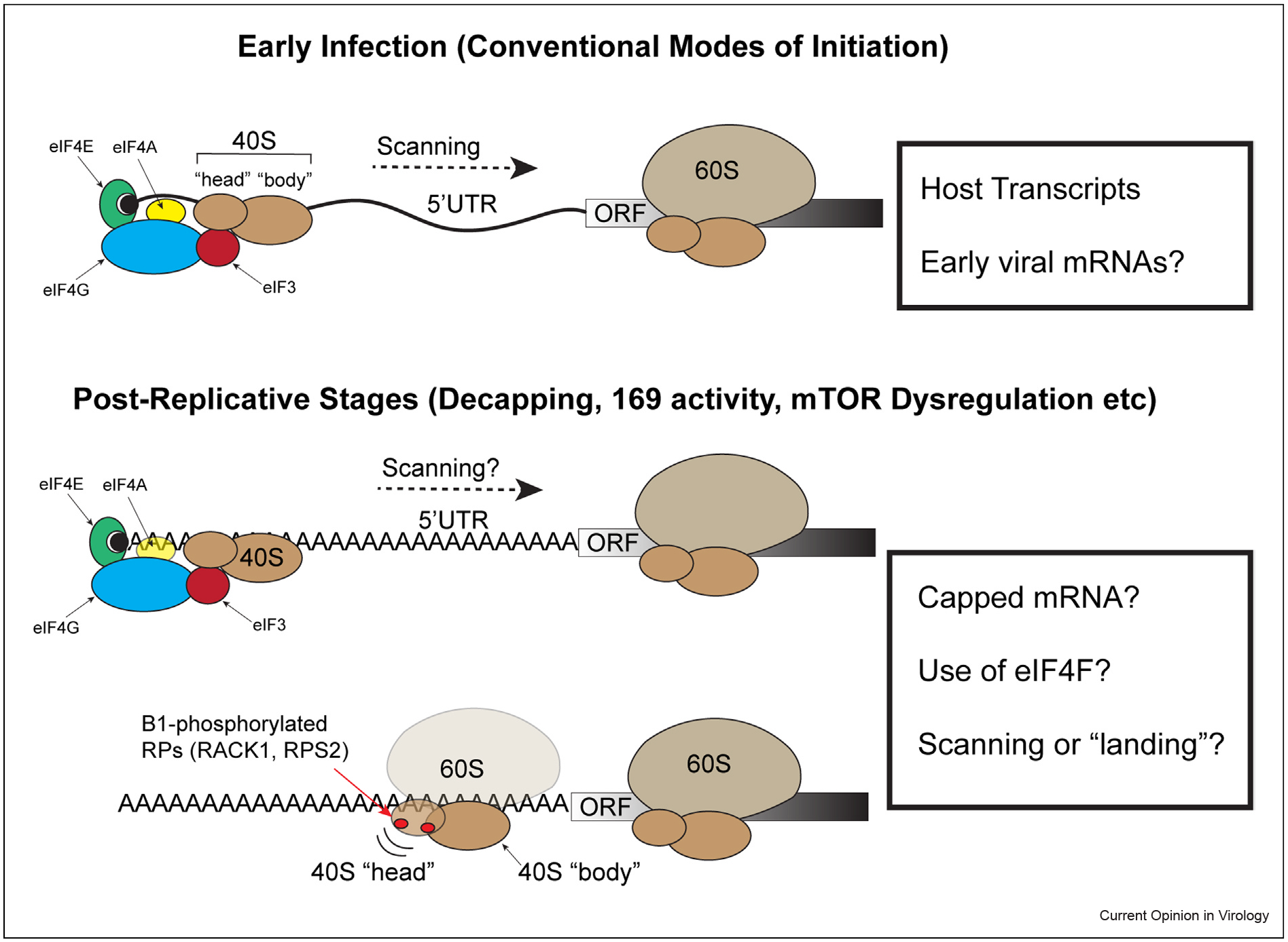
Illustration of key aspects of ribosome recruitment during early and postreplicative stages of poxvirus infection. Top: A typical host or early viral mRNA contains a 5’ 7-methyl-GTP ‘Cap’ (black circle) and an UTR that precedes the ORF. Recruitment of the ribosome to the correct end of the mRNA involves cap recognition by the eIF4E subunit of the eIF4F complex, which further consists of the scaffold protein eIF4G and the RNA helicase eIF4A. The eIF4G scaffold also binds the multi-subunit complex eIF3 that bridges to the 40S ribosomal subunit; the 40S complex contains 33 subunits that form ‘head’ and ‘body’ regions that can swivel as the 40S inspects the mRNA. The helicase activity of eIF4F facilitates 40S scanning of the UTR to find the start codon at which point the 60S ribosomal subunit joins to begin decoding the ORF. The length and structural complexity of the UTR influences both the degree of dependence on eIF4F helicase activity and translation efficiency of different mRNAs. While structurally similar and therefore likely to also require eIF4F and eIF3 activity, it has yet to be directly proven that early viral mRNAs are translated in a cap-dependent manner similar to their host. Bottom: Owing to the presence of viral proteins that drive decapping, mTOR dysregulation, and broader changes to the cell at later stages of infection, it remains unclear whether postreplicative viral mRNAs are capped or use eIFs for initiation, or whether they utilize mixed initiation strategies. The heterogeneous lengths and relatively short nature of the 5’ polyA-leaders found on postreplicative mRNAs also raises questions as to whether scanning is involved. Moreover, unique B1-driven phosphorylation events on small ribosomal proteins, in particular RACK1, alter the swivel motion of the 40S and reduce dependence of certain transcripts on the helicase activity of eIF4F. This likely supports cap-independent recruitment of the ribosome to the unstructured polyA-stretches that directly precede the start codon.
