Figure 2.
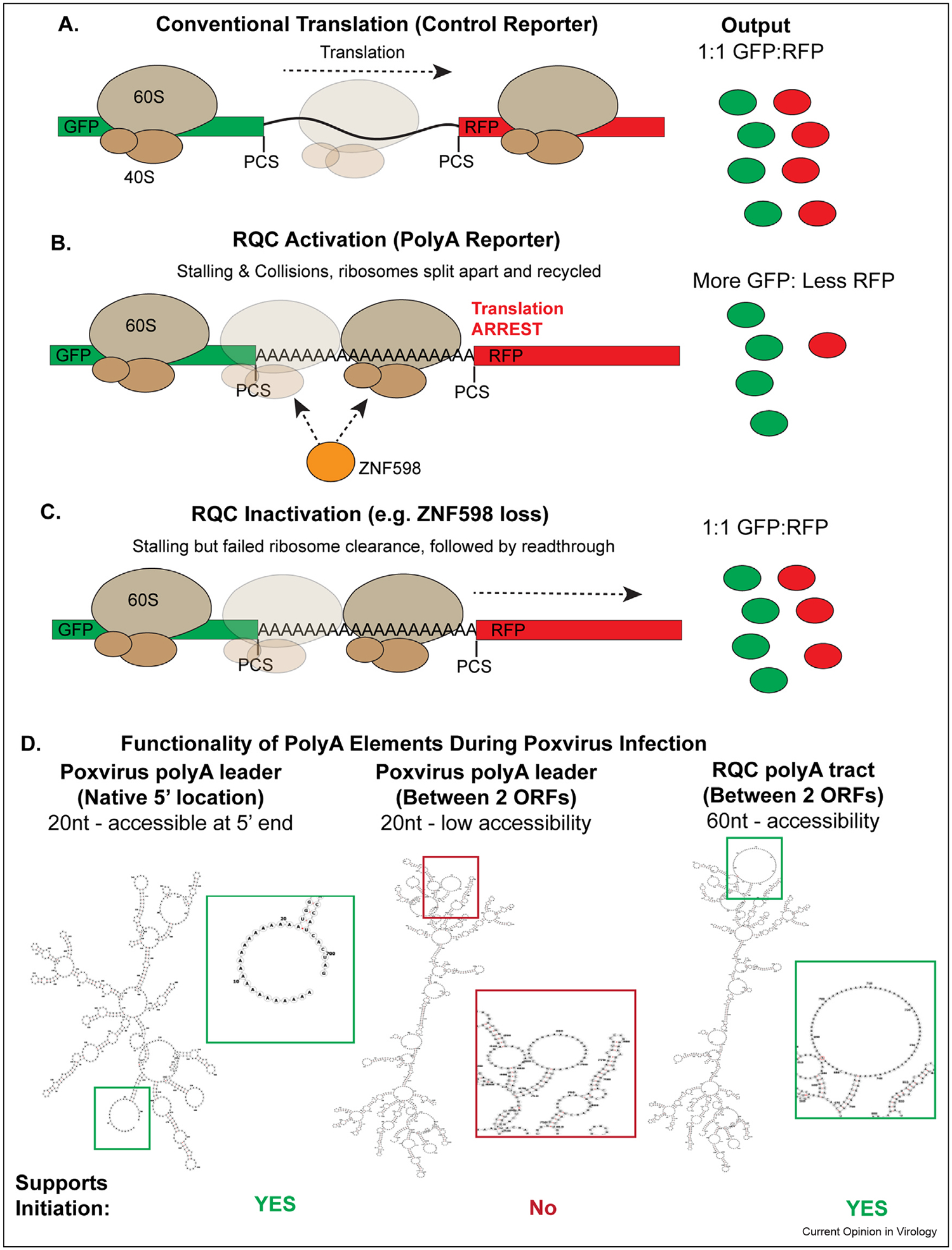
A model for RQC and polyA element activities during poxvirus infection. (a–c) RQC reporters consist of GFP and RFP (or mCherry) separated by a nucleotide test sequence. In addition, GFP and RFP are flanked by protease cleavage sites (PCS) so that GFP or RFP production are not interdependent and GFP can still be made when stalling and RQC occur. (a) Conventional translation of a reporter with a control linker sequence produces equal GFP and RFP. (b) The presence of a 60-nt polyA linker sequence causes ribosomes to stall and collide. ZNF598 senses collisions and through monoubiquitylation, triggers release of the stalled ribosomes to be recycled. As a result, cells make GFP but very little RFP due to the termination of translation of the reporter on the polyA sequence. (c) In the absence of RQC activity, such as with loss of ZNF598, ribosomes stall but eventually move through the polyA tract to continue translation of the internally polyadenylated mRNA. As a result, levels of GFP and RFP normalize to those of control reporters. While it remains unclear how RQC activity is stimulated during early poxvirus infection, the recycling of stalled ribosomes driven by RQC factors likely ensures a sufficient pool of active ribosomes to support the high levels of viral protein synthesis that occurs. (d) A model for differences in how polyA sequences and reporters behave in poxvirus-infected cells. Note that poxvirus polyA-leaders average 10–40 nts and their functionality as an IRES has been performed using representative 20-nt elements. By contrast, RQC reporters use 60-nt polyA-stretches to induce ribosome stalling. RNAfold (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi.) was used to model the effects of these fundamental differences in polyA lengths, using GFP as a model ORF. Boxed regions zoom in on the modeled polyA sequence for each panel. Left: Native poxvirus leaders are short but are located at the free 5’ end of the mRNA, making them readily accessible to ‘landing’ by poxvirus-modified ribosomes. Middle: The 20-nt poxvirus leader modeled between two GFP ORFs forms a small and likely poorly accessible region for ribosomes attempting to land internally. They therefore likely lack IRES functionality in bicistronic reporter assays. Right: The 60-nt polyA element used in RQC studies forms a large unstructured element that is likely more accessible to poxvirus-modified ribosomes to randomly initiate internally as well as normally at the 5’UTR, sustaining higher levels of RFP than GFP expression. While different reporter setups are used in different studies, the simplest explanation for the different functionalities observed to date is accessibility of the polyA tracts based on their sizes and locations.
