Abstract
High‐grade myofibroblastic sarcoma is a rare mesenchymal tumor with a high recurrence and metastatic rate. Few cases of high‐grade myofibroblastic sarcomas have been reported. Herein, we report a rare case of undifferentiated, high‐grade myofibroblastic sarcoma with an unclear primary site, initially presenting with oral symptoms. High‐grade myofibroblastic sarcoma was diagnosed following an excisional biopsy of a gingival tumor. After this excisional biopsy, systemic imaging revealed multiple metastases in the tonsil, lung, liver, kidney, and eye. The patient underwent two cycles of chemotherapy (doxorubicin). During follow‐up, the tumor progressed rapidly and metastasized to the skin of the head and neck. The patient expired three months after the initial examination.
Keywords: chemotherapy, immunohistochemical staining, multiple metastases, myofibroblastic sarcoma
Short abstract
This is an extremely rare case of myofibroblastic sarcoma that initially presented with symptoms in the oral cavity, without malignant findings. After tumor excision, multiple metastases were found throughout the body. This rare case provides valuable information to clinicians.
1. INTRODUCTION
Myofibroblastic sarcoma is a rare disease involving myofibroblasts or myofibroblastic differentiation, initially identified and reported by Mentzel et al. 1 It is widely reported that myofibroblastic sarcoma develops in the deep regions of the head and neck. 2 , 3 , 4 , 5 It is divided into low‐grade myofibroblastic sarcoma, included in the World Health Organization (WHO) classification, and high‐grade myofibroblastic sarcoma (HGMS), not included in the WHO classification. 2 , 3
This case report highlights HGMS of the gingiva in the oral cavity of a 66‐year‐old male. This is a rare case in which initial symptoms presented in the oral cavity, despite the presence of multiple metastases throughout the body.
2. CASE EXAMINATION
A 66‐year‐old man was referred by his private dental office for evaluation of a painless swelling in the right lower gum. The patient's family history was unremarkable. The patient had arterial hypertension, no allergies to medicine or food, and no history of smoking. The results of all laboratory tests were within normal limits. There was no clinical evidence of lymphadenopathy. Clinical examination showed a soft and pedunculated 15 × 20 mm tumor with a smooth surface and no erosion (Figure 1A). An orthopantomogram revealed an enlarged periodontal ligament of the first premolar tooth and well‐delineated bone resorption in the distal region of the first premolar tooth (Figure 2). The lesion, which had been growing for 4 weeks, was originally interpreted as an epulis derived from the right lower first premolar tooth. The first premolar tooth related to the tumor was considered to have a poor prognosis owing to remarkable alveolar bone resorption around the root.
FIGURE 1.
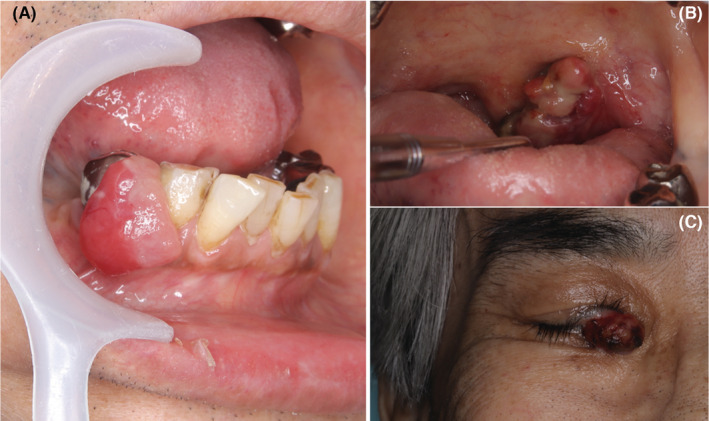
Soft and pedunculated tumor on the gum in the oral cavity (A). Cauliflower‐like shaped tumor in left tonsil (B). Fragile tumor in the eye similar to conjunctival pyogenic granuloma (C).
FIGURE 2.
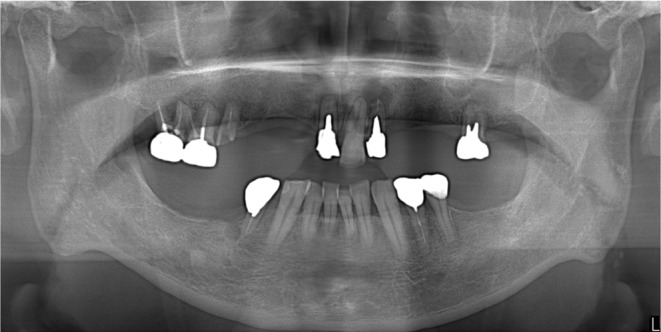
Panoramic radiograph at first examination.
3. DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
An excisional biopsy of the oral tumor was performed under local anesthesia. The first premolar tooth was extracted, and the alveolar bone around the tooth was removed. Macroscopically, the tumor was removed by a clear margin and had slightly hard elasticity. Notably, no necrosis was observed. Microscopic examination of the resected specimen showed that the tumor was composed of a diffuse and bundled proliferation of spindle‐shaped cells with edematous nuclei, increased cell density, and nuclear atypia (Figure 3). The mitotic rate was 10–30/10 high‐power fields. The Ki‐67 (MIB‐1) index was high, at approximately 37.1%. No lymphocyte infiltration, myxoid tissue, or vessel intervention was observed. Immunohistochemically, the tumor cells were positive for α‐smooth muscle actin, vimentin, CD34, and p53 (Figure 4) and negative for CAM5.2, S‐100 protein, and desmin. Immunohistochemical staining indicated differentiation into myofibroblasts, and the histological diagnosis was consistent with myofibroblastic sarcoma.
FIGURE 3.
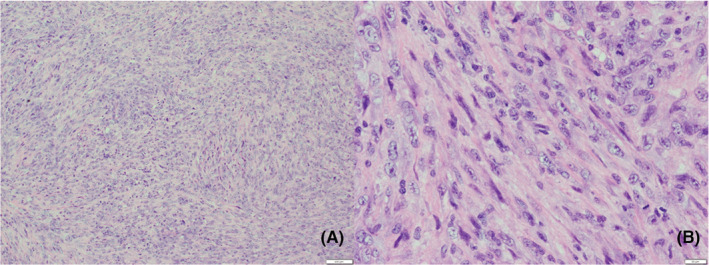
Diffuse and bundler proliferation pattern of spindle‐shaped cells with edematous nuclei, with increased cell density and nuclear atypia. Hematoxylin–eosin stain. Original magnification (A: 10×, B: 40×).
FIGURE 4.
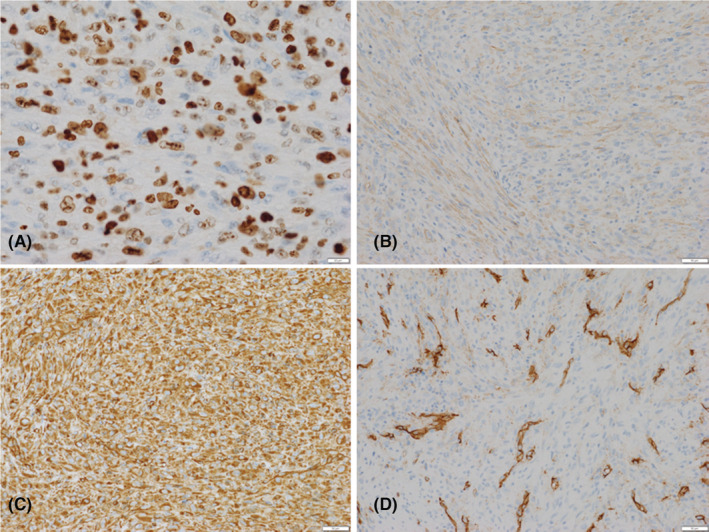
Immunohistochemical staining. Neoplastic cells strongly stained for MIB‐1 (A), α‐smooth muscle actin (B), vimentin (C), and CD 34 (D), and are focally positive.
After the excisional biopsy, the initial clinical follow‐up did not reveal any signs of recurrence in the oral cavity. A few weeks later, the patient complained of anorexia, weight loss, slight hemoptysis, and sore throat at his private clinic. No tumor was found in the colon on total colonoscopy. Multiple gastric ulcers and benign tumors were found on esophagogastroduodenoscopy. Computed tomography revealed multiple tumors in the liver, 15 cm above the left kidney tumor, and bilateral lung tumors (Figure 5). Biopsy of a tumor with a cauliflower‐like shape in the left tonsil revealed metastasis of myofibroblastic sarcoma to the pharynx. Furthermore, an excisional biopsy of a tumor, which had been clinically diagnosed as a conjunctival pyogenic granuloma, revealed metastasis of myofibroblastic sarcoma to the eye (Figure 1B,C).
FIGURE 5.
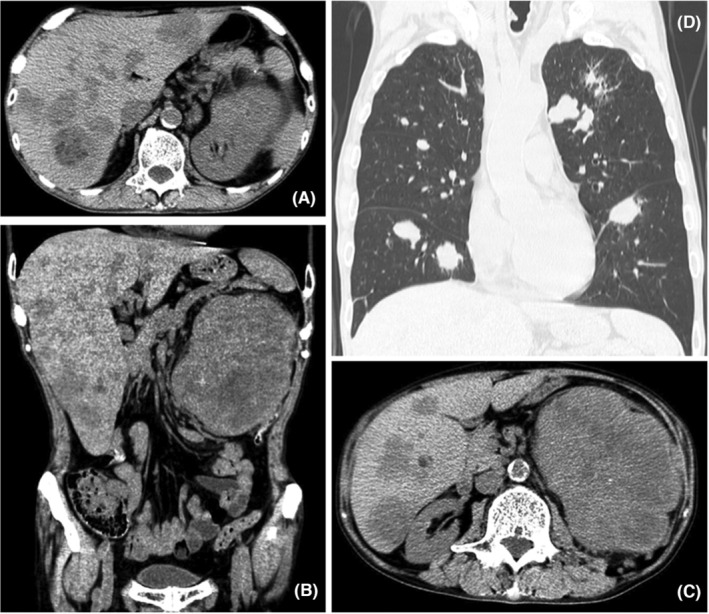
Computed tomography scan showing multiple metastases of myofibroblastic sarcoma in the liver, kidneys (A–C), and lungs (D).
4. OUTCOME AND FOLLOW‐UP
Although the initial symptom was observed in the oral cavity, the current case was considered to be that of multiple systemic metastases of myofibroblastic sarcoma, for which the primary site was unknown. After consultation with a multidisciplinary team, including a medical oncologist, the patient was administered chemotherapy with doxorubicin alone (60 mg/m2) every 21 days. Although we had considered continuing chemotherapy with doxorubicin alone or changing to eribulin administration (1.4 mg/m2) twice every 21 days, after two cycles of chemotherapy with doxorubicin alone, the tumor progressed rapidly and metastasized to the skin of the head and neck, and the patient died 3 months after the first examination.
5. DISCUSSION
Myofibroblastic sarcoma is a rare disease that predominantly develops in the deep soft tissues of the head and neck region, followed by the extremities, and then the trunk. 2 , 3 , 4 , 5 Myofibroblastic sarcoma is divided into low‐, intermediate‐, and high‐grade types according to cytomorphological analysis and immunophenotyping. The former two types are classified as low‐grade myofibroblastic sarcomas by the WHO. However, HGMS, also known as pleomorphic myofibrosarcoma, is not included in the WHO classification. 2 , 3 HGMS demonstrates aggressive growth and has higher recurrence and metastasis rates as compared to low‐grade myofibroblastic sarcoma. 2 , 4 Fisher reported 32% local recurrence and 68% metastasis among 22 morphologically confirmed cases. 6 In contrast, low‐grade myofibroblastic sarcoma has a local recurrence rate of 5%–10% and rare distant metastasis after wide‐margin excision. 6
The case presented here was positive for α‐SMA, vimentin, and CD53, and negative for cytokeratin AE1/AE3, desmin, and S‐100, which is consistent with immunohistochemical findings of myofibroblastic sarcoma reported in the literature. 1 , 2 , 3 In addition, the MIB‐1 index and mitotic activity, independent prognostic parameters for soft tissue sarcoma, were also high. Therefore, according to the Fédération Nationale Des Centres de Lutte Contre Le Cancer (FNCLCC) grading system, this case was classified as Grade 3 with a total score of 6, because the histological type indicated undifferentiated sarcoma without necrosis. 7 , 8
Given the rare incidence of myofibroblastic sarcoma, there have been few reports describing its etiology. However, complete surgical resection is the first choice for HGMS. Generally, a wide margin of at least 1–2 cm is recommended, because microscopically positive surgical margins are associated with a high risk of local recurrence, distant metastasis, and death. 2 , 3 , 4 In 46 cases of myofibroblastic sarcoma in the head and neck, Cai et al. reported four patients with HGMS who had undergone partial excision, experienced relapse, and died. 9
Additional treatments with adjuvant chemotherapy, radiotherapy, and surgical resection may be necessary to control the tumor. Koga et al. reported a case of HGMS in the pericardium that was treated with thoracotomy, followed by postoperative chemotherapy (doxorubicin and ifosfamide) and radiotherapy, in which the residual tumor gradually regressed, and no progression was noted until 6 months after surgery. 10 Giving priority to chemotherapy and radiotherapy over surgery as radical treatment remains controversial, but HGMS arising from the pleura has been reported to have good outcomes with only chemotherapy and radiotherapy. 3
In this case, additional surgery to ensure an adequately wide margin of resection of the oral lesion was considered; however, multiple metastases were present throughout the body when HGMS with an unknown primary site was diagnosed. The combination chemotherapy of doxorubicin and ifosfamide for unresectable advanced soft tissue sarcoma had a better response rate but frequently induced adverse events. 11 Therefore, for unresectable advanced HGMS, doxorubicin was administered alone as first‐line chemotherapy for advanced soft tissue sarcoma, in consideration of the patient's general condition. 11 However, the tumor grew aggressively, and the patient died after two cycles of chemotherapy with doxorubicin alone.
There is limited knowledge regarding cases of HGMS for which the initial symptoms appear in the gingiva of the oral cavity, despite metastases throughout the body. This case report presents a rare case of HGMS, for which the primary site was unknown and whose initial symptoms were found in the oral cavity. This would be beneficial for clinicians who come across such similar rare cases.
AUTHOR CONTRIBUTIONS
Takeshi Harada treated the patient and wrote this paper. Takeshi Togawa, Hiroki Miyamoto, and Yutaka Matsushita treated the patient and collected the patient's data. Yukimasa Hatachi treated the patient.
FUNDING INFORMATION
We have no sources of funding for our manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that no conflict of interest exists.
PATIENT CONSENT STATEMENT
Because the patient was deceased, written informed consent was obtained from the patient's elder sister to publish this report in accordance with the journal's patient consent policy. However, all treatments and examinations were conducted with the consent of the patient.
ACKNOWLEDGMENTS
We thank the medical staff of Kansai Rosai Hospital who cared for the patient.
Harada T, Togawa T, Miyamoto H, Matsushita Y, Hatachi Y. A unique case of high‐grade myofibroblastic sarcoma initially presenting with oral symptoms. Clin Case Rep. 2023;11:e7218. doi: 10.1002/ccr3.7218
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low‐grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228‐1238. doi: 10.1097/00000478-199810000-00008 [DOI] [PubMed] [Google Scholar]
- 2. Wen J, Zhao W, Li C, Shen JY, Wen TF. High‐grade myofibroblastic sarcoma in the liver: a case report. World J Gastroenterol. 2017;23:7054‐7058. doi: 10.3748/wjg.v23.i38.7054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao R, Wang J, Zhang H, Chi Y, Bi N. High‐grade myofibroblastic sarcoma of the pleura: a case report and literature review. Thorac Cancer. 2002;11:3011‐3014. doi: 10.1111/1759-7714.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fletcher CD, Bridge J, Hogendoorn P, Mertens F. WHO Classification of Tumors of Soft Tissue and Bone. 4th ed. IARC Press; 2013:15‐18. [Google Scholar]
- 5. Coffin CM, Alaggio R. Fibroblastic and myofibroblastic tumors in children and adolescents. Pediatr Dev Pathol. 2012;15:127‐180. doi: 10.2350/10-12-0944-PB.1 [DOI] [PubMed] [Google Scholar]
- 6. Fisher C. Myofibroblastic malignancies. Adv Anat Pathol. 2004;11:190‐201. doi: 10.1097/01.pap.0000131773.16130.aa [DOI] [PubMed] [Google Scholar]
- 7. Neuville A, Chibon F, Coindre JM. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology. 2014;46:113‐120. doi: 10.1097/PAT.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 8. Hasegawa T. Histological grading and MIB‐1 labeling index of soft‐tissue sarcomas. Pathol Int. 2007;57:121‐125. doi: 10.1111/j.1440-1827.2006.02068.x [DOI] [PubMed] [Google Scholar]
- 9. Cai C, Dehner LP, El‐Mofty SK. In myofibroblastic sarcomas of the head and neck, mitotic activity and necrosis define grade: a case study and literature review. Virchows Arch. 2013;463:827‐836. doi: 10.1007/s00428-013-1494-1 [DOI] [PubMed] [Google Scholar]
- 10. Koga S, Ikeda S, Urata J, et al. Primary high‐grade myofibroblastic sarcoma arising from the pericardium. Circ J. 2008;72:337‐339. doi: 10.1253/circj.72.337 [DOI] [PubMed] [Google Scholar]
- 11. Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomized controlled phase 3 trial. Lancet Oncol. 2014;15:415‐423. doi: 10.1016/S1470-2045(14)70063-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


