Abstract
Background
Rheumatoid arthritis (RA)-associated interstitial lung disease (ILD) is common in patients with RA and leads to significant morbidity and mortality. No randomized, placebo-controlled data are available that support the role of immunosuppression to treat RA-associated ILD, despite being widely used in clinical practice.
Research Question
How does immunosuppression impact pulmonary function trajectory in a multisite retrospective cohort of patients with RA-associated ILD?
Study Design and Methods
Patients with RA who started treatment for ILD with mycophenolate, azathioprine, or rituximab were identified retrospectively from five ILD centers. Change in lung function before and after treatment was analyzed using a linear spline mixed-effect model with random intercept. Prespecified secondary analyses examined the impact of radiologic pattern of ILD (ie, usual interstitial pneumonia [UIP] vs non-UIP) on treatment trajectory.
Results
Two hundred twelve patients were included in the analysis: 92 patients (43.4%) were treated with azathioprine, 77 patients (36.3%) were treated with mycophenolate mofetil, and 43 patients (20.3%) were treated with rituximab. In the combined analysis of all three agents, an improvement in FVC % predicted was found after 12 months of treatment compared with the potential 12-month response without treatment (+3.90%; P ≤ .001; 95% CI, 1.95-5.84). Diffusing capacity of the lungs for carbon monoxide (Dlco) % predicted also improved at 12 months (+4.53%; P ≤ .001; 95% CI, 2.12-6.94). Neither the UIP pattern of ILD nor choice of immunosuppressive agent significantly impacted the pulmonary function trajectory on immunosuppression.
Interpretation
Immunosuppression was associated with an improved trajectory in FVC and Dlco compared with the pretreatment pulmonary function trajectory. Prospective, randomized trials are required to validate these findings.
Key Words: azathioprine, interstitial lung disease, mycophenolate, rituximab, rheumatoid arthritis
Take-home Points.
Study Question: How does immunosuppression impact pulmonary function trajectory in a multisite retrospective cohort of patients with rheumatoid arthritis (RA)-associated interstitial lung disease (ILD)?
Results: In 212 patients with RA-associated ILD from five centers, the addition of azathioprine, mycophenolate mofetil, or rituximab led to improved pulmonary function test results at 1 year when compared with pretreatment pulmonary function trend before additional immunosuppression. The presence of a fibrotic ILD pattern did not significantly impact this immunosuppression response.
Interpretation: Our data support the current paradigm of primary, initial immunosuppression for treatment of RA-associated ILD regardless of underlying ILD pattern. However, the limitations of observational and retrospective study design highlight the urgency for randomized, placebo-controlled trials for these patients.
Rheumatoid arthritis (RA) is common, affecting 1% of the general population.1 The development of RA-associated interstitial lung disease (ILD) causes significant morbidity and mortality and affects anywhere from 10% to 40% of those with RA.2 Despite the high incidence and clinical significance of RA-associated ILD, data from randomized, placebo-controlled trials powered specifically for patients with RA-associated ILD to support the primary use of immunosuppression are sparse.3 This gap in treatment-based data for RA-associated ILD is even more pressing given the clinical, genetic, and histologic overlaps between RA-associated ILD and idiopathic pulmonary fibrosis (IPF),4 where seminal randomized, placebo-controlled trials found that immune suppression in patients with IPF caused increased morbidity and mortality.5
Historically, treatment approaches in RA-associated ILD primarily are based on lung-directed, steroid-sparing immunosuppression with agents such as: azathioprine, mycophenolate mofetil (MMF), or rituximab. This immunosuppressive-based treatment practice in RA-associated ILD has been extrapolated from data in scleroderma lung disease or has been derived from observational clinical experiences in patients with RA-associated ILD.6, 7, 8, 9, 10, 11, 12 Interest has been increasing in the use of novel antifibrotic treatment in progressive, fibrotic phenotypes of ILD, including patients with RA-associated ILD, for which results from initial trials showed similar outcomes as those for IPF.13
Given the well-accepted role of immunosuppression in RA-associated ILD, we hypothesized that patients with RA-associated ILD would show a favorable response to immunosuppression treatment based on pulmonary physiology trajectories and that the presence of the interstitial pneumonia (UIP) pattern would hinder that response.
Study Design and Methods
Study Population
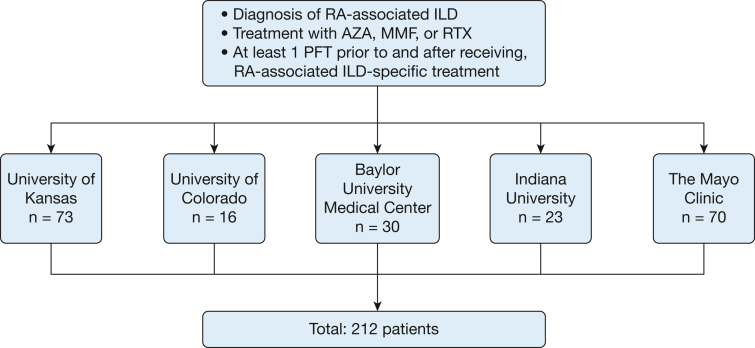
This cohort included patients from five tertiary ILD centers: University of Kansas Medical Center, University of Colorado Denver, Indiana University, Baylor University Medical Center, and the Mayo Clinic Rochester (Fig 1). Patients were identified from prospectively enrolled ILD databases at the University of Colorado Denver and Mayo Clinic Rochester and from retrospective review of sequential medical records from the ILD clinics at the University of Kansas Medical Center, Indiana University, and Baylor University Medical Center. Patients were included if they had received a diagnosis of RA-associated ILD from their evaluating ILD physician; had received treatment with either azathioprine, MMF, or rituximab for this diagnosis; and had at least one value for FVC or diffusing capacity of the lungs for carbon monoxide (Dlco) before and after treatment initiation.
Figure 1.
Flow diagram showing cohort formation. AZA = azathioprine; ILD = interstitial lung disease; MMF = mycophenolate mofetil; RTX = rituximab; PFT = pulmonary function test; RA = rheumatoid arthritis.
Patients at the University of Kansas Medical Center, University of Colorado Denver, Indiana University, and Baylor University Medical Center (n = 142) underwent the most recent CT scan of the chest before treatment (if available), reviewed at a central reading location (University of Kansas Medical Center). Two thoracic-trained radiologists (K. P. and C. M. W.) were masked to the rest of the study variables and assessed the high-resolution CT scans for usual UIP pattern based on the most recent Fleischner Society guidelines; instances of discordant initial interpretation were resolved by consensus. Patients from Mayo Clinic Rochester (n = 70) underwent UIP determination based on the interpretation of CT scans by two ILD physicians (M. B. and T. M.) using the same UIP criteria, and discrepant reads were resolved by a third ILD physician (J. H. R.). For these analyses and for comparability with previous studies, we limited our analysis time frame to include only PFT measures obtained ± 24 months from treatment initiation.
Data was extracted from the medical record including clinical demographics, RA disease features, and joint-specific RA treatment history. RA-associated ILD-specific treatment history (including start and stop dates, doses, discontinuation, addition of or change in therapy, drug tolerability, and adverse events) also were extracted. All pulmonary function test results obtained before and after RA-associated ILD-specific treatment was initiated also were collected.
Statistical Analysis
The choice of initial treatment (azathioprine vs MMF vs rituximab) was used as the primary predictor variable for this study. For a specific lung function measurement, the time variable was calculated as the date at which the measurement was obtained minus the date immunosuppression treatment was started, and this difference was converted to months. Thus, time = 0 represents treatment initiation, and a negative time means that lung function was measured before treatment. A linear spline mixed-effect model with a random intercept including FVC % predicted (and Dlco % predicted) as the dependent variable was fitted with time = 0 (date of ILD treatment initiation) as the knot using all eligible patients.14,15 As independent variables, the model included both time and a spline variable equal to time if time was > 0 or 0 otherwise. The predicted mean response after 12 months of treatment was compared with the potential mean response that would have been observed if the pretreatment trend had continued. Thus, we were comparing the after-treatment lung function trajectory over time with a trajectory extrapolated from the before-treatment trajectory.16 In this sense, participants served as their own control subjects.
To examine whether UIP, defined as definite or probable UIP, modified the predicted time trend for FVC (or Dlco) % predicted, an additional random intercept model that included interactions of UIP with time and the spline variable was fitted. If the interaction between UIP and the spline variable was significant, we concluded that UIP modified the trajectory of the response. To compare the effects of azathioprine, MMF, or rituximab on FVC (or Dlco) % predicted trajectory, dummy variables representing treatments were created. Then main effects of treatments and interactions between treatments and time and spline variables were added to the random intercept model. To adjust for potential drug choice bias, all of the above models were weighted with the inverse of propensity scores calculated with a multinomial logistic model of the probability of prescribing either azathioprine, MMF, or rituximab.17 The model was built through backward selection, and the selected variables used to calculate the propensity scores were age at ILD treatment initiation, ethnicity, pulmonary hypertension, prednisone use, and sex. The nonselected variables were race, baseline RA treatment, gastroesophageal reflux disease, ever smoking status, OSA diagnosis and UIP pattern. All statistical tests were two-sided and conducted at a P = .05 significance level. Statistical analyses were conducted with SAS version 9.4 software (SAS Institute).
Results
Description of Study Cohort
Baseline characteristics are included in Table 1. Most patients were women (62.3%) and had a history of ever smoking (59.7%) with a mean ± SD age of 63.5 ± 14.6 years at the time of treatment initiation. Most of the patients, 90.3%, showed seropositive results for either anticyclic citrullinated peptide (72.3%) or rheumatoid factor (80.3%). Most patients showed a non-UIP pattern on high-resolution CT imaging (59.6%). The mean FVC % predicted and Dlco % predicted at the time of RA-associated ILD treatment initiation was 66.9 ± 18.2% predicted and 50.5 ± 18.1% predicted, respectively. At the time of RA-associated ILD treatment initiation, 67.9% of patients were receiving baseline prednisone with mean dose of 13.2 ± 15.8 mg daily. Most patients (69.3%) were receiving preexisting disease-modifying antirheumatic drug therapy at the time of RA-associated ILD treatment initiation (Table 1). Characteristics by study site are provided in e-Table 1.
Table 1.
Patient Characteristics
| Characteristic | Data (N = 212 Patients) |
|---|---|
| Age, y | |
| At treatment initiation | 63.5 ± 14.6 |
| At RA diagnosis | 56.5 ± 13.7 |
| Male sex | 80 (37.7) |
| Non-White racea | 34 (16.3) |
| Non-Hispanicb | 140 (67.6) |
| Ever smokerc | 126 (59.7) |
| Seropositive findingsd | 187 (90.3) |
| Anti-CCPe | 136 (72.3) |
| RFf | 159 (80.3) |
| RA-associated UIPg | 80 (40.4) |
| GERDh | 146 (69.5) |
| Pulmonary hypertensioni | 67 (32.7) |
| OSAj | 86 (42.2) |
| Median follow-up (after treatment initiation), mo | 27.5 |
| Pulmonary function at treatment initiation | |
| FVC % predicted | 66.9 ± 18.2 |
| Dlco % predicted | 50.5 ± 18.1 |
| Baseline treatment when ILD-specific treatment initiated | |
| Prednisone | 144 (67.9) |
| Prednisone dose, mg | 13.2 ± 15.8 |
| Hydroxychloroquine | 56 (26.4) |
| Leflunomide | 29 (13.7) |
| Methotrexate | 25 (11.8) |
| Infliximab | 15 (7.1) |
| Sulfasalazine | 13 (6.1) |
| Etanercept | 12 (5.7) |
| Abatacept | 10 (4.7) |
| Adalimumab | 8 (3.8) |
| Golimumab | 4 (1.9) |
| Tofacitinib | 4 (1.9) |
| Certolizumab | 2 (0.9) |
| Nintedanib | 2 (0.9) |
| Pirfenidone | 1 (0.5) |
| ILD treatment initiated | |
| Azathioprine | 92 (43.4) |
| Azathioprine daily dose, mg | 120.1 (50-300) |
| Mycophenolate | 77 (36.3) |
| Mycophenolate daily dose, mg | 1,964.3 (500-3,000) |
| Rituximabk | 43 (20.3) |
Data are presented as No. (%), mean ± SD, or mean (range). CCP = cyclic citrullinated peptide; Dlco = diffusing capacity of the lungs for carbon monoxide; GERD = gastroesophageal reflux disease; ILD = interstitial lung disease; RA = rheumatoid arthritis, RF = rheumatoid factor; UIP = usual interstitial pneumonia.
Race information was not available for three patients.
Ethnicity information was not available in five patients.
Ever smoking status was unknown for one patient.
Seropositivity status was not available for five patients.
Anti-CCP status was not available for 24 patients.
RF status was not available for 14 patients.
RA-associated UIP status was not available for 14 patients.
GERD status was not available for two patients.
Pulmonary hypertension status was not available for seven patients.
OSA status was not available for eight patients.
Rituximab universal starting dose was 1,000 mg on day 1 infusion.
Two hundred twelve patients met the inclusion and exclusion criteria; however, because patients could contribute FVC or Dlco measurements or both, some patients contributed only FVC measurements (n = 203) and some contributed only Dlco measurements (n = 194). A total of 865 FVC measurements were obtained between –24 and 24 months: 333 before treatment and 532 at or after treatment. Seven hundred fourteen Dlco measurements were included in the analysis: 275 Dlco measurements before treatment and 439 Dlco measurements at or after treatment. Years of observation included in this study were 2000 through 2021 and the median follow-up time was 27.5 months.
Immunosuppression Impact on Longitudinal Change in Lung Function
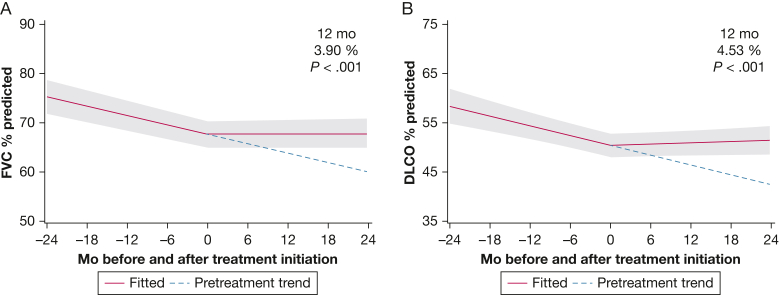
Investigation of the collective impact of immunosuppression (ie, azathioprine, MMF, or rituximab) on lung function trajectory after ILD treatment initiation demonstrated that the mean FVC % predicted after 12 months of treatment was significantly higher (+3.90%; P ≤ .001; 95% CI, 1.95-5.84) when compared with the potential mean response that would have been observed if the predicted pretreatment trend of FVC % predicted had continued (Fig 2A). Note that the effect of treatment on change in lung function after 12 months of treatment was estimated as the difference in the observed change and the counterfactual change that would have been expected had treatment not been initiated.16
Figure 2.
A, Line graph showing impact of immunosuppression on predicted trajectory of FVC % predicted. The pretreatment trend in FVC is shown from time –24 months to time 0, when RA-associated ILD-specific treatment was initiated. The pretreatment trend (blue dotted line) is projected forward from time 0 to +24 months and compared with the observed FVC trend after treatment initiation. After 12 months of treatment, significant increase in FVC % predicted was achieved compared with the projected trend without treatment (+3.90; P < .001; 95% CI, 1.95-5.84). Gray shading indicates 95% CIs. B, Line graph showing impact of immunosuppression on Dlco. A significant increase in Dlco % predicted after 12 months of treatment was found compared with the projected trend without treatment (+4.53%; P ≤ .001; 95% CI, 2.12-6.94). Dlco = diffusing capacity of the lungs for carbon monoxide.
We found that the effect of immunosuppression on the predicted Dlco % predicted response after 12 months of treatment was associated with a significant improvement (+4.53%; P ≤ .001; 95% CI, 2.12-6.94) when compared with the potential mean response that would have been observed had the pretreatment trend continued (Fig 2B).
Impact of ILD Pattern on Treatment Response
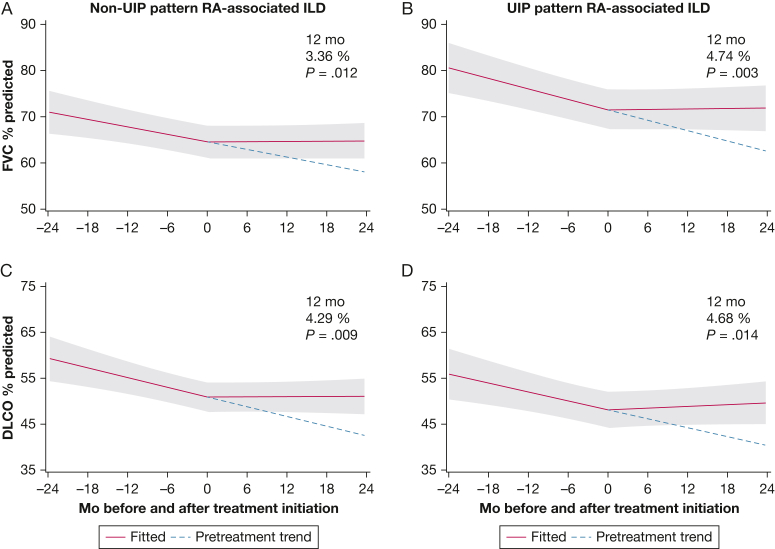
When added to the linear spline mixed-effect model used for our primary outcome and adjusted with inverse propensity score weights, the presence of definite or probable UIP did not impact treatment response significantly at 12 months for FVC % predicted (P = .5). According to the interaction model, both the non-UIP and UIP groups showed significant improvements in FVC % predicted compared with pretreatment trends (+3.36% [P = 0.012; 95% CI, 0.74-5.98] and +4.74% [P = .003; 95% CI, 1.64-7.83], respectively); however, the presence of UIP did not impact the response trajectory significantly over time (P = .5, interaction between UIP and spline variable) (Fig 3A, 3B). We observed similar findings with Dlco % predicted (P = .9 for interaction) (Fig 3C, 3D).
Figure 3.
A, Line graph showing the trajectory of FVC % predicted response before and after immunosuppression treatment (azathioprine, mycophenolate mofetil, or rituximab) for patients with RA without a definite or probable UIP pattern on chest imaging. Compared with the potential response without treatment, the change after 12 months of treatment was +3.36% (P = .012; 95% CI, 0.74-5.98). B, Line graph showing the FVC response for patients with RA with UIP pattern (+4.74%; P = .003; 95% CI, 1.64-7.83). In the overall model, the presence of definite or probable UIP did not significantly impact treatment response at 12 months for FVC % predicted based on the interaction analysis (P = .506). C, Line graph showing the Dlco response for patients with RA without UIP pattern (4.29%; P = .009; 95% CI, 1.08-7.50). D, Line graph showing the Dlco response for patients with RA with UIP pattern (4.68%; P = .014; 95% CI, 0.96-8.40). In the overall model, the presence of definite or probable UIP did not significantly impact treatment response at 12 months for Dlco % predicted based on the interaction analysis (P = .9). Dlco = diffusing capacity of the lungs for carbon monoxide; ILD = interstitial lung disease; RA = rheumatoid arthritis; UIP = interstitial pneumonia.
In a sensitivity analysis, we found that the method of UIP determination (ie, radiologist interpretation vs ILD physician interpretation [Mayo Clinic Rochester]) showed no impact on treatment outcomes. Specifically, UIP pattern did not modify the effect of immune suppression treatment on FVC % predicted or Dlco % predicted significantly in separate models fitted either with the patients from Mayo Clinic Rochester or patients from the other sites.
Choice of ILD Therapy (Azathioprine vs MMF vs Rituximab) on Treatment Response
Using a random intercept linear spline mixed-effect model adjusting for inverse of propensity scores as weights, we found no significant differences in FVC % predicted response between treatments (azathioprine vs MMF [P = .8] and azathioprine vs rituximab [P = .8] [e-Fig 1A-1C]; e-Table 2 shows a comparison of patient characteristics by drug choice [azathioprine vs MMF vs rituximab]). Similarly, although the predicted Dlco % predicted response at 12 months for rituximab, relative to the pretreatment trend, seemed to be stronger than the relative predictions for MMF and azathioprine (+6.73% vs +3.67% vs +1.93%, respectively), we found no significant differences between treatments (azathioprine vs MMF [P = .595] and azathioprine vs rituximab [P = .1]) (e-Fig 1D-1F).
Tolerability of Azathioprine, MMF, and Rituximab
Each drug was well tolerated in most patients (Table 2), with 35 patients (16.5%) reporting adverse events and 16 patients (7.5%) discontinuing treatment because of adverse events. The most common of these adverse events reported were nonspecific GI symptoms seen in 10 patients (4.7%). Other adverse events reported were diarrhea in nine patients (4.2%), recurrent infections in seven patients (3.3%), cytopenia in six patients (2.8%), and elevated liver enzymes in three patients (1.4%). Based on treatment choice, 12 patients receiving azathioprine discontinued therapy because of adverse events (13.0%) vs three patients receiving MMF (3.9%) and one patient receiving rituximab (1.3%).
Table 2.
Medication Adverse Events and Treatment Alterations
| Variable | Total Cohort (N = 212) | Azathioprine (n = 92) | MMF (n = 77) | Rituximab (n = 43) |
|---|---|---|---|---|
| Reported adverse event | 35 (16.5) | 18 (19.6) | 12 (15.6) | 5 (11.6) |
| GI upset | 9 (4.2) | 3 (3.3) | 5 (6.5) | 1 (2.3) |
| Elevated liver enzymes | 3 (1.4) | 3 (3.3) | 0 | 0 |
| Cytopenia | 6 (2.8) | 3 (3.3) | 2 (2.6) | 1 (2.3) |
| Recurrent infections | 7 (3.3) | 4 (4.3) | 2 (2.6) | 1 (2.3) |
| Nonspecific symptoms | 10 (4.7) | 5 (5.4) | 3 (3.9) | 2 (4.7) |
| Treatment stopped because of adverse event | 16 (7.5) | 12 (13.0) | 3 (3.9) | 1 (2.3) |
| Immunosuppression treatment alterations during follow-up | ||||
| Treating clinician impression of progression of RA joint disease | ||||
| Added therapy | 12 (5.7) | 5 (5.4) | 6 (7.8) | 1 (2.3) |
| Switched or discontinued therapy | 6 (2.8) | 1 (1.1) | 2 (2.6) | 3 (7.0) |
| Treating clinician impression of ILD progression | ||||
| Added therapy | 15 (7.1) | 7 (7.6) | 7 (9.1) | 1 (2.3) |
| Switched or discontinued therapy | 2 (0.9) | 1 (1.1) | 0 | 1 (2.3) |
Data are presented as No. (%). ILD = interstitial lung disease; MMF = mycophenolate mofetil; RA = rheumatoid arthritis.
During the period of follow-up, treating clinicians added therapy to the initial ILD regimen in 15 patients (7.1%) and switched or discontinued the initial therapy in two patients (0.9%) because of ILD progression. The initial treatment regimen was augmented in 12 patients (5.7%) and switched or discontinued for six patients (2.8%) because of concern for RA joint disease progression.
Discussion
In this multisite, retrospective study of RA-associated ILD treatment, we demonstrated that treatment with immunosuppression using azathioprine, MMF, or rituximab was associated with significant improvement in FVC % predicted and Dlco % predicted at 12 months after treatment initiation compared with values predicted from pretreatment trends. In our a priori subgroup analysis, we found no difference in lung function trajectory based on high-resolution CT scan-derived UIP pattern. Choice of specific therapy (azathioprine vs MMF vs rituximab) also did not significantly impact FVC % predicted or Dlco % predicted treatment response. All three therapies seemed to be well tolerated given the low rates of discontinuation and recorded adverse effects in a retrospective chart review.
These findings support previous single-center observational reports exploring the role of MMF or azathioprine in connective tissue disease-related ILD (including patients with RA-associated ILD) as well as randomized controlled data in scleroderma-associated ILD.6, 7, 8, 9,11 In contrast to several of these studies, our study had a larger sample size and a multicenter design focused exclusively on patients with RA-associated ILD. To our knowledge, this is also the first study to assess the impact of UIP pattern on immunosuppression treatment response in RA-associated ILD.
Concern has been expressed regarding the addition of immunosuppression for patients with RA-associated ILD with UIP pattern given the negative impact of immunosuppression in IPF on mortality, particularly as it pertains to regimens that include azathioprine.5 For this reason, we assessed the impact of radiologic UIP in our model and found no interaction between radiologic UIP and immunosuppression on lung function after treatment. Decline in FVC has been an important prognostic marker for increased mortality in both IPF and RA-associated ILD.18,19 Our data support the initial use of immunosuppression in RA-associated ILD regardless of ILD pattern given this lack of significant impact of UIP pattern on the FVC trajectory; however, important caveats exist. For instance, we did not assess the impact of immunosuppression on mortality or hospitalizations in this cohort because of the study design, and this essential question remains best answered in a randomized study protocol. Additionally, the RA-associated ILD cohort showed a slightly lower proportion of UIP pattern (38%) than what has been reported in other observational cohorts of RA-associated ILD,20, 21, 22 suggesting potential selection bias in the cohort regarding UIP pattern. Although radiologist-determined UIP pattern is specific for UIP on histopathologic examination, those that do not meet criteria for radiologic UIP still could show UIP on histopathologic analysis, which could result in misclassification.23
We did not observe differential outcomes in either FVC % or Dlco % predicted after treatment based on specific immunosuppressive agent. These results were obtained after correcting for lack of randomization by weighting the model with inverse propensity scores based on treatment choice. Our data were collected from patient experiences from 2000 through 2021, and therefore primarily are based on patient experiences before the Food and Drug Administration approval of nintedanib for progressive-fibrotic ILD13 or on a recently published randomized controlled trial of pirfenidone in patients with RA-associated ILD.24 Further randomized trials are needed to determine the best treatment strategy or sequence of treatment approaches for these patients given the role of prescription bias in retrospective studies such as this.
The study design limits our ability to comment on the impact of immunosuppressive treatment on other outcomes such as RA disease control, quality of life, hospitalizations, or mortality, which are of most importance to patients. Although immortal time bias is not directly applicable to our results because we have not commented on survival, it is important to point out that the nature of this study design selects for patients who were both (1) robust enough to tolerate therapy based on clinician impression and (2) able to undergo at least one follow-up PFT whose results were obtained clinically. We also acknowledge the possibility of referral bias in our data given that each of the five sites are tertiary ILD referral centers, which likely enriches for a population of patients with RA-associated ILD with more severe disease features and who potentially are more likely to experience progression.
Interpretation
Finally, given recent emerging data on the role of RA disease severity impact on incident RA-associated ILD and RA-associated ILD prognosis,25,26 we believe that future prospective studies of patients with RA-associated ILD should evaluate the impact of immunosuppression on RA disease severity and ILD outcomes. Multidisciplinary discussion between rheumatologists and pulmonologists that weighs this balance of immunosuppression for both articular and lung disease remains the standard of care for patients with RA-associated ILD.
Our study design included patients with fewer than two FVC or Dlco measurements obtained before treatment. As a result, we selected random effects models for our primary analysis because they are robust to data imbalances caused by missing data.14 We confirmed the results of our primary analyses in additional sensitivity analyses that included only the patients who had two or more measurements that were obtained before treatment. Specifically, in a combined analysis of all three agents that included only the 95 patients who provided two or more FVC measurements obtained before treatment, a significant improvement in FVC % predicted after 12 months of treatment was found compared with the potential 12-month response without treatment (+4.05%; P = .001; 95% CI, 1.64-6.46). Similarly, Dlco % predicted also improved at 12 months in the analysis that included only the 81 patients who provided two or more Dlco measurements obtained before treatment (+3.43%; P = .025; 95% CI, 0.44-6.41).
Our data support the current paradigm of primary, initial immunosuppression for treatment of RA-associated ILD. However, the limitations of observational and retrospective study design highlight the urgency for randomized, placebo-controlled trials in RA-associated ILD.
Funding/Support
S. M. M. is supported by National Institute of General Medical Sciences [Grant P20GM13423].
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: S. M. M. served as prinicipal author and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. S. M. M. and J. S. L. conceived of the study design and formulated the goals and aims of the original proposal. S. M. M., J. S. L., J. D., and F. J. D. developed the statistical design methodology and models, J. D. and F. J. D. carried out implementation of the statistical coding and computational analysis and created the first draft of the statistical analysis section of the manuscript. S. M. M., M. B., T. M., M. M., J. K., N. S. I., R. D. B., J. M. D., S. K. M., and J. H. R. performed data collection and investigation process. K. P. and C. M. W. generated data via blinded radiographic interpretation of computed tomography images. M. K. D. and M. B. H. provided assistance to initial study design parameter design and preparation of manuscript. S. M. M. wrote the manuscript with J. S. L. as supervisor, however, all authors discssed the results and contributed to the final manuscript.
Other contributions: The authors thank Melanie Basham, Haylie Lengel, and Victoria Small.
Additional information:e-Figure and e-Tables are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Gabriel S.E. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz T., Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottin V., Lega JC, Coury F, Nasser M. A call for evidence in connective tissue diseases-associated interstitial lung disease. Joint Bone Spine. 2021;89(1) doi: 10.1016/j.jbspin.2021.105274. [DOI] [PubMed] [Google Scholar]
- 4.Matson S., Lee J., Eickelberg O. Two sides of the same coin? A review of the similarities and differences between idiopathic pulmonary fibrosis and rheumatoid arthritis associated interstitial lung disease. Eur Respir J. 2020;57(5):2002533. doi: 10.1183/13993003.02533-2020. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G., Anstrom KJ, King TE, Jr., Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashkin D.P., Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A., Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–646. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldham J.M., Lee C, Valenzi E, et al. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir Med. 2016;121:117–122. doi: 10.1016/j.rmed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saketkoo L.A., Espinoza L.R. Rheumatoid arthritis interstitial lung disease: mycophenolate mofetil as an antifibrotic and disease-modifying antirheumatic drug. Arch Intern Med. 2008;168(15):1718–1719. doi: 10.1001/archinte.168.15.1718. [DOI] [PubMed] [Google Scholar]
- 10.Brito Y., Glassberg M.K., Ascherman D.P. Rheumatoid arthritis-associated interstitial lung disease: current concepts. Curr Rheumatol Rep. 2017;19(12):79. doi: 10.1007/s11926-017-0701-5. [DOI] [PubMed] [Google Scholar]
- 11.Md Yusof MY, Kabia A, Darby M, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford) 2017;56(8):1348–1357. doi: 10.1093/rheumatology/kex072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panopoulos S.T., Sfikakis P.P. Biological treatments and connective tissue disease associated interstitial lung disease. Curr Opin Pulm Med. 2011;17(5):362–367. doi: 10.1097/MCP.0b013e3283483ea5. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty K.R., Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 14.Hedeker D., Gibbons R.D. Wiley; 2006. Longitudinal Data Analysis. [Google Scholar]
- 15.James G., Witten D., Hastie T., Tibshirani R. 2nd ed. Springer; 2021. An Introduction to Statistical Learning: With Applications in R. [Google Scholar]
- 16.Morisset J., Johannson KA, Vittinghoff E, et al. Use of mycophenolate mofetil or azathioprine for the management of chronic hypersensitivity pneumonitis. Chest. 2017;151(3):619–625. doi: 10.1016/j.chest.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodward M. 3rd ed. Chaphan & Hall/CRC; 2014. Epidemiology. Study Design and Data Analysis. [Google Scholar]
- 18.Du Bois R.M., Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184(12):1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 19.Solomon J.J., Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 20.Kim E.J., Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 21.Solomon J.J., Saravanan V, Nisar M, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) Respir Med. 2013;107(8):1247–1252. doi: 10.1016/j.rmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Kelly C.A., Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics—a large multicentre UK study. Rheumatology (Oxford) 2014;53(9):1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 23.Assayag D., Elicker BM, Urbania TH, et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology. 2014;270(2):583–588. doi: 10.1148/radiol.13130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon J.J., Danoff SK, Woodhead FA, et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med. 2023;11(1):87–96. doi: 10.1016/S2213-2600(22)00260-0. [DOI] [PubMed] [Google Scholar]
- 25.Sparks J.A., He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019;71(9):1472–1482. doi: 10.1002/art.40904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks R., Baker JF, Yang Y, et al. The impact of disease severity measures on survival in U.S. veterans with rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford) 2022;61(12):4667–4677. doi: 10.1093/rheumatology/keac208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.