Abstract
Background
The healthy donor effect (HDE) is a selection bias caused by the health criteria blood donors must meet. It obscures investigations of beneficial/adverse health effects of blood donation and complicates the generalizability of findings from blood donor cohorts. To further characterize the HDE we investigated how self‐reported health and lifestyle are associated with becoming a blood donor, lapsing, and donation intensity. Furthermore, we examined differences in mortality based on donor status.
Study Design and Methods
The Danish National Health Survey was linked to the Scandinavian Donations and Transfusions (SCANDAT) database and Danish register data. Logistic‐ and normal regression was used to compare baseline characteristics and participation. Poisson regression was used to investigate future donation choices. Donation intensity was explored by the Anderson‐Gill model and Poisson regression. Mortality was investigated using Poisson regression.
Results
Blood donors were more likely to participate in the surveys, OR = 2.45 95% confidence interval (2.40–2.49) than non‐donors. Among survey participants, better self‐reported health and healthier lifestyle were associated with being or becoming a blood donor, donor retention, and to some extent donation intensity, for example, current smoking conveyed lower likelihood of becoming a donor, OR = 0.70 (0.66–0.75). We observed lower mortality for donors and survey participants, respectively, compared with non‐participating non‐donors.
Conclusion
We provide evidence that blood donation is associated with increased likelihood to participate in health surveys, possibly a manifestation of the HDE. Furthermore, becoming a blood donor, donor retention, and donation intensity was associated with better self‐reported health and healthier lifestyles.
Keywords: blood donors, donation behavior, donor health, healthy donor effect, survey participation
1. INTRODUCTION
Surveys have repeatedly demonstrated that blood donors enjoy better health and lower mortality than their non‐donating peers in the general population. 1 , 2 , 3 This has caused speculations about beneficial health effects of repeated whole blood donations, for example, prevention of cardio‐vascular disease by iron store depletion. 4 , 5 , 6
However, the so‐called healthy donor effect (HDE) challenges observational studies of potential health consequences of blood donations. HDE is a selection bias that arises because blood donors must continuously meet certain health criteria to ensure their own health and that of transfusion recipients. 7 , 8
The HDE complicates not only assessment of beneficial health effects of blood donations, but may also obscure adverse health consequences of repeated blood donation.
The HDE suggests that individuals entering the blood donor population a priori would be healthier than non‐donors and that returning blood donors would, on average, be healthier than lapsing donors. Additionally, high‐frequency donors might also be healthier than less frequently donating donors. 7 , 9 , 10 , 11
It is unclear from current literature how much blood donations contribute to the donors' superior health and how much the donors' superior health contributes to donation activity.
Thus, while studies have found that self‐reported health and lifestyle is predictive of donation cessation within a donor population, 9 no investigations have detailed if health‐behaviors and self‐reported health is predictive of becoming a blood donor, and whether these factors vary with time since blood donation activity.
Few studies have moved beyond comparisons of morbidity and mortality patterns between donors and non‐donors. 3 , 12 The largest of such investigations is a German cross‐sectional survey of health patterns and ‐behaviors among 10,318 non‐donors, 1012 lapsed‐, and 1153 active donors.
The German study showed that the three groups differed regarding modifiable lifestyle and self‐perceived health, active donors being the most, non‐donors the least healthy, and lapsed donors resembling active donors more than non‐donors. 1
To further the understanding of the relationship between health and blood donation, we linked large population‐based surveys with nation‐wide information on blood donors to explore the association between self‐reported health and modifiable lifestyle factors and risk of becoming, being, and having been a blood donor, respectively, and whether these factors were associated with differences in longevity between donors and non‐donors.
2. MATERIALS AND METHODS
We used data from The Danish National Health Surveys (DNHS) 2010 and 2013, the Scandinavian Donations and Transfusions (SCANDAT) database, and various Danish registers. 13 , 14 , 15 , 16
DNHS are a series of nation‐wide health and lifestyle surveys carried out in the Danish population at regular intervals. 13 , 16 It features a standardized questionnaire, answered online or on paper, which includes information on anthropometrics, lifestyle, and health; including the Short Form 12 (SF‐12) questionnaire. 17 SF‐12 summarizes self‐reported health in two dimensions, expressed as physical component score (PCS) and mental component score (MCS). Both PCS and MCS range from 0 to 100, with higher scores indicating better health.
The 2010 and 2013 surveys were both carried out in six mutually exclusive random population sub‐samples; one for each of the five administrative regions in Denmark and a national sample. 16 Overall, 298,550 and 300,450 individuals ≥16 years of age were invited in 2010 and 2013, respectively. A total of 177,639 (59.5%) and 162,283 (54.0%) individuals partially or fully completed the questionnaires. 32,348 persons were invited in both 2010 and 2013; for these individuals only the 2010 survey data were included in the present study. The two surveys were both conducted from late January/early February through April in their respective years.
SCANDAT is a Danish‐Swedish database containing data on blood donors and donations as well as blood transfusions and transfused patients. The database contains more than 25 million donation records and 21 million transfusion records from 1968 (Sweden) and 1980 (Denmark) till November 2017. 14
Since 1968, all individuals living in Denmark have been issued unique personal identification numbers, used by Danish authorities for administrative purposes. This includes the Civil Registration System, which continuously monitors the vital status of all Danish citizens as well as nation‐wide socio‐demographic registers maintained by Statistics Denmark. 18
Using the personal identification number as key, we linked DNHS and SCANDAT to identify blood donors among individuals invited to participate in DNHS. Next, we linked the entire DNHS cohort to the Civil Registration System to ascertain their vital status and to registers at Statistics Denmark for information on socio‐demographic data. 15 , 18
A complete case analysis was performed, excluding participants with missing values for personal identification number, age, sex, body mass index (BMI), SF‐12 scores, region of residence, smoking habits, diet score, weekly alcohol intake, or physical activity. Outliers were removed for Short Form 12 scores outside the upper or lower bounds of scoring. 17 , 19 Following the above procedures and combining DNHS 2010 and 2013 data, 321,986 participants were available for analysis (Figure S1).
2.1. Statistics
Following standard conventions, blood donors were considered lapsed (former donors) if the most recent donation was >2 years before time of the survey, otherwise they were deemed active (current donors). In 2010 and 2013, individuals were not allowed to donate blood at age 67+ years, and consequently we stopped follow‐up at this time whenever relevant. For analytical simplicity, the date of DNHS invitation and interview (baseline) for participants was set to January 1st 2010 or 2013.
Analyses of participation and death were conducted in the full material of participants and non‐respondents using sex, birth data, invitation date, calendar time, and donation history as predictors. All other analyses were restricted to subsets of participants with all relevant information available. Usually three estimates were provided: (a) a crude estimate, (b) one adjusted for age and sex, and (c) one further adjusted for region (of residence at time of invitation), attained highest education (short (basic school), medium, long (12+ years)), and income (percentile of personal annual income). For the predictors PCS, MCS, and BMI we further adjusted for smoking status, diet, alcohol consumption, and physical activity.
The following lifestyle exposures were considered: binge drinking (consuming ≥5 × 12 g alcohol on one occasion monthly or more often), low risk alcohol consumption (<7 × 12 g alcohol per week in females, <14 × 12 g alcohol per week in males), moderate risk alcohol consumption, high risk alcohol consumption (>14 × 12 g alcohol per week in females, >21 × 12 g alcohol per week in males), smoking status (current, former, never), extreme physical activity (training hard regularly/several times a week to participate in sports competitions), high physical activity (participate in physical sports, physically demanding gardening or similar ≥4 h a week), light physical activity (light locomotion such as walking, cycling, and similar <4 h a week), passive physical activity (reading, watching TV, or similar), and diet (healthy, average, unhealthy).
We used logistic regression analyses to investigate participation and dichotomous characteristics at baseline. We used normal regression to compare BMI, PCS, and MCS at baseline. We used Poisson regression to predict becoming a donor (for non‐donors at baseline), lapsing donation (for active donors at baseline), and dying (participants and non‐respondents) in the period from invitation to end of study (December 31, 2019). Mortality was further analyzed in the same period by stratified Cox regression with sex and year of birth defining the strata. Finally, we assessed (in active donors) whether baseline characteristics predicted intensity of donation using the Andersen‐Gill model which allows for multiple outcome events. That is, as soon as we observed an outcome event (blood donation) we (re‐)started follow‐up for the next outcome event. This was performed using Poisson regression. Follow‐up was definitively stopped at the latest recorded donation or the latest recorded donation before lapsing for the first time during follow‐up. This was based on the experience of Ullum et al. 10 that donation frequency is a habitual thing that does not gradually decline before lapsing. All statistical analyses were performed using SAS version 9.4 and R version 3.5.1. 20
3. RESULTS
3.1. Participation
Of 566,652 individuals invited to DNHS, 62,086 (11%) had at least one whole blood donation registered in SCANDAT. Among 322,015 participants, 46,348 (14.4%) had a history of blood donation, while among 244,637 non‐respondents this number was 15,738 (6.4%).
This corresponded to blood donors being more than twofold (odds ratio (OR) = 2.45 96% confidence interval [2.40–2.49]) more likely to participate in the survey than non‐donors.
Both among blood donors and non‐donors, participation rates were higher among women than men and increased with age (Table 1), particularly pronounced among men (data not shown). Female blood donors were marginally more likely to participate (OR = 2.57 [2.50–2.65]) than male blood donors (OR = 2.43 [2.37–2.49]).
TABLE 1.
Cohort characteristics
| Participants | Non‐respondents | |||||
|---|---|---|---|---|---|---|
| Donor status | Active | Lapsed | Non | Active | Lapsed | Non |
| (N = 19,465) | (N = 26,882) | (N = 275,659) | (N = 5754) | (N = 9984) | (N = 228,899) | |
| Sex | ||||||
| Female | 10,220 (52.5%) | 14,735 (54.8%) | 149,226 (54.1%) | 2542 (44.2%) | 4783 (47.9%) | 106,219 (46.4%) |
| Male | 9245 (47.5%) | 12,147 (45.2%) | 126,433 (45.9%) | 3212 (55.8%) | 5201 (52.1%) | 122,680 (53.6%) |
| Age (years) | ||||||
| Mean (SD) | 43.0 (13.1) | 52.6 (14.1) | 51.4 (18.7) | 39.3 (12.8) | 48.8 (14.1) | 46.8 (20.2) |
| Age groups | ||||||
| 15–24 | 2089 (10.7%) | 349 (1.3%) | 32,124 (11.7%) | 976 (17.0%) | 259 (2.6%) | 40,641 (17.8%) |
| 25–34 | 3512 (18.0%) | 2980 (11.1%) | 25,071 (9.1%) | 1242 (21.6%) | 1481 (14.8%) | 31,967 (14.0%) |
| 35–44 | 4431 (22.8%) | 5012 (18.6%) | 40,238 (14.6%) | 1440 (25.0%) | 2347 (23.5%) | 37,960 (16.6%) |
| 45–54 | 5056 (26.0%) | 5840 (21.7%) | 49,101 (17.8%) | 1269 (22.1%) | 2433 (24.4%) | 37,950 (16.6%) |
| 55–64 | 3756 (19.3%) | 6212 (23.1%) | 53,102 (19.3%) | 733 (12.7%) | 1899 (19.0%) | 31,362 (13.7%) |
| 65–74 | 621 (3.2%) | 5302 (19.7%) | 47,036 (17.1%) | 94 (1.6%) | 1192 (11.9%) | 23,542 (10.3%) |
| 75–84 | 0 (0%) | 1134 (4.2%) | 22,813 (8.3%) | 0 (0%) | 329 (3.3%) | 16,051 (7.0%) |
| 85+ | 0 (0%) | 53 (0.2%) | 6174 (2.2%) | 0 (0%) | 44 (0.4%) | 9426 (4.1%) |
| PCS | ||||||
| Mean (SD) | 54.6 (6.18) | 51.3 (8.99) | 50.0 (10.2) | ‐ | ‐ | ‐ |
| Missing | 920 (4.7%) | 2332 (8.7%) | 33,800 (12.3%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
| MCS | ||||||
| Mean (SD) | 51.9 (8.15) | 51.4 (9.25) | 50.2 (9.90) | ‐ | ‐ | ‐ |
| Missing | 920 (4.7%) | 2332 (8.7%) | 33,800 (12.3%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
| Binge drinking | ||||||
| Yes | 6717 (34.5%) | 6637 (24.7%) | 68,670 (24.9%) | ‐ | ‐ | ‐ |
| No | 11,937 (61.3%) | 18,294 (68.1%) | 168,410 (61.1%) | ‐ | ‐ | ‐ |
| Missing | 811 (4.2%) | 1951 (7.3%) | 38,579 (14.0%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
| Alcohol consumption | ||||||
| High risk | 1900 (9.8%) | 2875 (10.7%) | 31,700 (11.5%) | ‐ | ‐ | ‐ |
| Moderate risk | 3473 (17.8%) | 4345 (16.2%) | 41,285 (15.0%) | ‐ | ‐ | ‐ |
| Low risk | 13,083 (67.2%) | 17,255 (64.2%) | 157,445 (57.1%) | ‐ | ‐ | ‐ |
| Missing | 1009 (5.2%) | 2407 (9.0%) | 45,229 (16.4%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
| Smoking | ||||||
| Current | 3160 (16.2%) | 4979 (18.5%) | 60,841 (22.1%) | ‐ | ‐ | ‐ |
| Former | 5226 (26.8%) | 9336 (34.7%) | 84,173 (30.5%) | ‐ | ‐ | ‐ |
| Never | 10,833 (55.7%) | 12,204 (45.4%) | 123,823 (44.9%) | ‐ | ‐ | ‐ |
| Missing | 246 (1.3%) | 363 (1.4%) | 6822 (2.5%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
| Physical activity | ||||||
| Extreme | 1022 (5.3%) | 663 (2.5%) | 12,183 (4.4%) | ‐ | ‐ | ‐ |
| High | 6438 (33.1%) | 6440 (24.0%) | 56,676 (20.6%) | ‐ | ‐ | ‐ |
| Light | 10,298 (52.9%) | 16,192 (60.2%) | 153,898 (55.8%) | ‐ | ‐ | ‐ |
| Passive | 1459 (7.5%) | 2975 (11.1%) | 42,901 (15.6%) | ‐ | ‐ | ‐ |
| Missing | 248 (1.3%) | 612 (2.3%) | 10,001 (3.6%) | 5754 (100%) | 9984 (100%) | 228,899 (100%) |
Note: Descriptive statistics for the combined cohort from the Danish National Health Survey 2010 and 2013. The data shown was after linking to SCANDAT and removing individuals with Short form 12 scores: physical component score (PCS) and mental component score (MCS), outside the reference range (0–100).
Adjusting for age and sex we found that each additional donation increased the odds of participation; OR = 1.007 (1.004–1.009) in active donors and OR = 1.010 (1.008–1.012) in lapsed donors.
3.2. Demographic characteristics at baseline
At time of the survey, both lapsed and active donors differed from non‐donors on several characteristics and generally with a smaller magnitude for lapsed than for active donors but the same direction of differences (Tables 1, 2, 3).
TABLE 2.
Active versus non‐donor as predictors of baseline characteristics among the Danish National Health Survey participants
| Baseline characteristic | OR (95% CI) crude | OR (95% CI) adjusted for sex and age | OR (95% CI) further adjusted |
|---|---|---|---|
| BMI: body mass index a | −.06 (−.13–0.01) | 0.20 (0.14–0.27) | 0.21 (0.14–0.27) |
| PCS: SF‐12 physical component score a | 3.57 (3.42–3.71) | 2.51 (2.38–2.65) | 1.31 (1.18–1.44) |
| MCS: SF‐12 mental component score a | 1.31 (1.16–1.45) | 1.93 (1.78–2.07) | 1.35 (1.21–1.50) |
| BMI < 18.5 (thin) | 0.30 (0.25–0.36) | 0.24 (0.20–0.29) | 0.35 (0.29–0.42) |
| BMI 18.5 ≤ BMI < 25 (normal) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BMI 25 ≤ BMI < 30 (overweight) | 1.02 (0.98–1.05) | 1.18 (1.14–1.23) | 1.16 (1.12–1.20) |
| BMI 30 ≤ BMI (obese) | 0.80 (0.76–0.84) | 0.92 (0.87–0.97) | 1.00 (0.95–1.06) |
| PCS < 40 | 0.25 (0.23–0.27) | 0.30 (0.27–0.33) | 0.44 (0.40–0.48) |
| PCS 40 ≤ PCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| PCS 60 ≤ PCS | 1.18 (1.12–1.25) | 1.00 (0.94–1.05) | 0.98 (0.93–1.04) |
| MCS < 40 | 0.58 (0.55–0.62) | 0.55 (0.52–0.58) | 0.66 (0.63–0.70) |
| MCS 40 ≤ MCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| MCS 60 ≤ MCS | 0.83 (0.79–0.87) | 1.05 (1.00–1.10) | 1.19 (1.13–1.25) |
| Alcohol consumption: high risk | 0.67 (0.63–0.71) | 0.66 (0.62–0.70) | 0.81 (0.77–0.86) |
| Alcohol consumption: moderate | 0.92 (0.88–0.96) | 0.94 (0.90–0.98) | 1.04 (1.00–1.09) |
| Alcohol consumption: low risk | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Binge drinking: yes | 1.31 (1.27–1.36) | 1.07 (1.03–1.10) | 1.15 (1.11–1.19) |
| Binge drinking: no | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Smoking: current | 0.59 (0.56–0.62) | 0.60 (0.58–0.63) | 0.67 (0.64–0.70) |
| Smoking: former | 0.71 (0.69–0.74) | 0.87 (0.84–0.91) | 0.88 (0.84–0.91) |
| Smoking: never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: extreme | 1.21 (1.13–1.30) | 0.82 (0.76–0.88) | 0.97 (0.90–1.04) |
| Physical activity: high | 1.56 (1.50–1.61) | 1.38 (1.34–1.44) | 1.27 (1.23–1.32) |
| Physical activity: light | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: passive | 0.64 (0.60–0.68) | 0.62 (0.58–0.66) | 0.66 (0.62–0.70) |
Note: The odds ratios (OR) denote increased or decreased likelihood among active donors as compared to non‐donors. The reference range for both the SF‐12 physical component score (PCS) and SF‐12 mental component score (MCS) was 0–100. Higher scores indicate better physical health for PCS and better mental health for MCS. Further adjusted ORs are adjusted for region (of residence at time of invitation), attained highest education (short (basic school), medium, long (12+ years)), and income (percentile of personal annual income). In addition, the predictors PCS, MCS, and BMI were also adjusted for smoking status, diet, alcohol consumption, and physical activity.
Not ORs but difference in mean value.
TABLE 3.
Lapsed versus non‐donor as predictors of baseline characteristics among the Danish National Health Survey participants
| Baseline characteristic | OR (95% CI) crude | OR (95% CI) adjusted for sex and age | OR (95% CI) further adjusted |
|---|---|---|---|
| BMI: body mass index a | 0.58 (0.52–0.64) | 0.49 (0.43–0.56) | 0.42 (0.36–0.49) |
| PCS: SF‐12 physical component score a | 0.71 (0.58–0.85) | 1.24 (1.12–1.36) | 0.52 (0.40–0.64) |
| MCS: SF‐12 mental component score a | 0.84 (0.71–0.98) | 0.60 (0.47–0.73) | 0.25 (0.12–0.38) |
| BMI < 18.5 (thin) | 0.42 (0.36–0.48) | 0.43 (0.37–0.50) | 0.55 (0.48–0.64) |
| BMI 18.5 ≤ BMI < 25 (normal) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BMI 25 ≤ BMI < 30 (overweight) | 1.19 (1.16–1.23) | 1.18 (1.14–1.21) | 1.15 (1.11–1.19) |
| BMI 30 ≤ BMI (obse) | 1.26 (1.21–1.32) | 1.23 (1.18–1.29) | 1.28 (1.22–1.33) |
| PCS < 40 | 0.78 (0.74–0.81) | 0.68 (0.65–0.72) | 0.85 (0.81–0.89) |
| PCS 40 ≤ PCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| PCS 60 ≤ PCS | 0.90 (0.85–0.95) | 1.01 (0.95–1.07) | 1.02 (0.97–1.08) |
| MCS < 40 | 0.83 (0.80–0.87) | 0.84 (0.81–0.88) | 0.96 (0.92–1.01) |
| MCS 40 ≤ MCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| MCS 60 ≤ MCS | 1.13 (1.09–1.18) | 1.04 (1.00–1.08) | 1.10 (1.05–1.14) |
| Alcohol consumption: high risk | 0.77 (0.73–0.81) | 0.77 (0.74–0.81) | 0.87 (0.82–0.91) |
| Alcohol consumption: moderate | 0.92 (0.89–0.96) | 0.89 (0.85–0.92) | 0.93 (0.89–0.96) |
| Alcohol consumption: low risk | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Binge drinking: yes | 0.86 (0.84–0.89) | 0.98 (0.95–1.01) | 1.02 (0.98–1.05) |
| Binge drinking: no | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Smoking: current | 0.80 (0.77–0.84) | 0.80 (0.77–0.83) | 0.87 (0.84–0.91) |
| Smoking: former | 1.12 (1.09–1.16) | 1.04 (1.00–1.07) | 1.04 (1.01–1.08) |
| Smoking: never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: extreme | 0.50 (0.46–0.55) | 0.63 (0.57–0.69) | 0.74 (0.68–0.81) |
| Physical activity: high | 1.04 (1.00–1.07) | 1.12 (1.08–1.16) | 1.06 (1.02–1.09) |
| Physical activity: light | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: passive | 0.78 (0.75–0.82) | 0.80 (0.76–0.84) | 0.84 (0.80–0.88) |
Note: The odds ratios (OR) denote increased or decreased likelihood among lapsed donors as compared to non‐donors. The reference range for both the SF‐12 physical component score (PCS) and SF‐12 mental component score (MCS) was 0–100. Higher scores indicate better physical health for PCS and better mental health for MCS. Further adjusted ORs were adjusted for region (of residence at time of invitation), attained highest education (short (basic school), medium, long (12+ years)), and income (percentile of personal annual income). In addition, the predictors PCS, MCS, and BMI were also adjusted for smoking status, diet, alcohol consumption, and physical activity.
Not ORs but difference in mean value.
Donors generally reported better health than non‐donors as measured by PCS and MCS. By design, the numbers in the tables are for main effects only. To illustrate that these effects are universal we have drawn smoothed curves of mean PCS and MCS by age, sex, and donor status (Figures 1 and 2). Changes in PCS and MCS for each additional donation adjusted for age and sex were; ΔPCS = −0.002 (−0.009–0.004) (active donors), ΔPCS = 0.021 (0.012–0.031) (lapsed donors), ΔMCS = 0.020 (0.011–0.029) (active donors), and ΔMCS = 0.034 (0.024–0.044) (lapsed donors).
FIGURE 1.
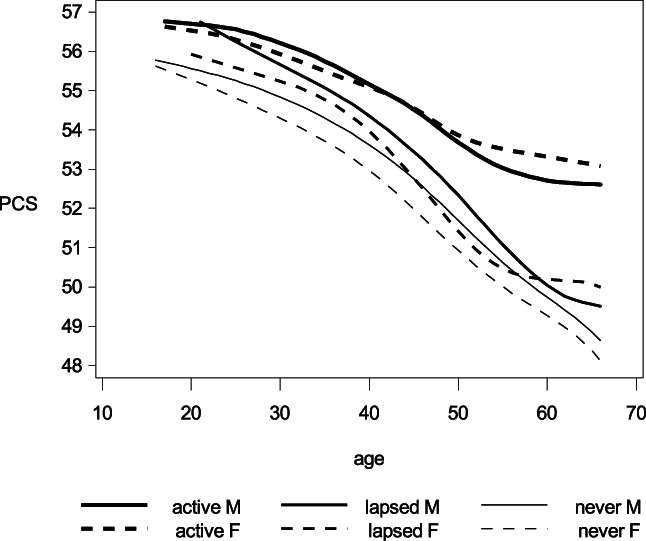
Smoothed curves for the mean short form 12 physical component score (PCS) by age, sex, and donor status. PCS can range from 0 to 100. Higher scores corresponds to better overall health. Line weight denote donor status: Active donors, lapsed donors, or never/non‐donors. The solid and dashed lines depicts mean PCS for males and females, respectively.
FIGURE 2.
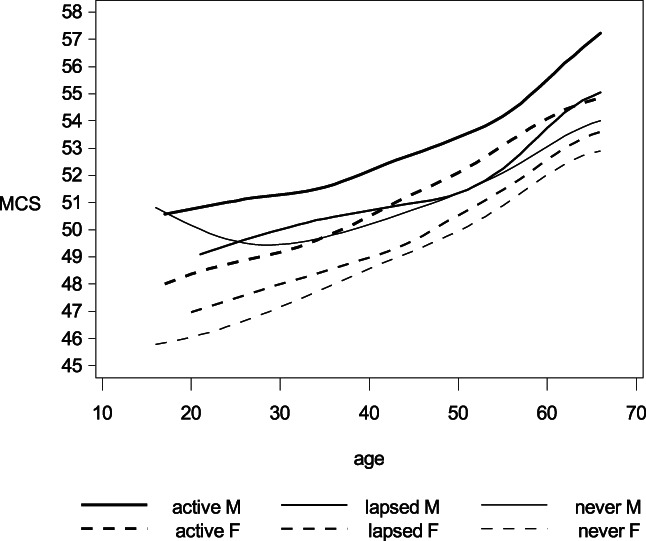
Smoothed curves for the mean short form 12 mental component score (MCS) by age, sex, and donor status. MCS can range from 0 to 100. Higher scores corresponds to better overall health. Line weight denote donor status: Active donors, lapsed donors, or never/non‐donors. The solid and dashed lines depicts mean MCS for males and females, respectively.
BMI was a little higher on average among donors than non‐donors, probably reflecting higher BMI in the center of the distribution. The low occurrence of thin donors (BMI < 18.5) was expected due to donor screening criteria, while there were more overweight (25 ≤ BMI < 30) (OR = 1.15–1.16) donors than non‐donors. Obesity (BMI ≥ 30) was more prevalent among lapsed donors (OR = 1.28) than among non‐donors, while there was no such overrepresentation among active donors (OR = 1.00).
Generally, blood donors were more physically active than non‐donors and fewer donors were inactive. Both current and former smoking was less common in active donors than in non‐donors; in lapsed donors this was only observed for current smoking.
The results regarding alcohol consumption did not fit a simple monotone pattern. Binge drinking was slightly more common in active donors than non‐donors (OR = 1.15 [1.11–1.19]), while this was not seen for lapsed donors (OR = 1.02 [0.98–1.05]). Likewise, moderate alcohol consumption was slightly more common in active donors (OR = 1.04 [1.00–1.09]) and slightly less common in lapsed donors (OR = 0.93 [0.89–0.96]) than in non‐donors.
3.3. Predictors of becoming blood donor and donor lapse
Next, we examined becoming a donor, among non‐donors at survey, and lapsing donation, among active donors at survey, and report hazard ratios (Tables 4 and 5) for the association of the same predictors as in (Tables 2 and 3) with these new outcomes. Generally, we saw that factors promoting recruitment into the donor corps also predicted donor retention and vice versa. Hence, increased PCS, MCS, and BMI generally predicted becoming donor and donor retention with the exception that very healthy donors (PCS ≥ 60 or MCS ≥ 60) did not obey the implied rules. We observed the expected association with physical activity (from Tables 2 and 3), that is, more physical activity was associated with increased likelihood of becoming a donor and donor retention. Extreme physical activity was a predictor of becoming a donor, but overall irrelevant as predictor of donor retention. Current smoking was negatively associated with entry into the donor population and was a predictor of lapsing donation. The patterns for alcohol consumption were also as expected (from Tables 2 and 3), with the exception that binge drinking was not associated with lapsing donation (HR = 1.00 [0.96–1.04]).
TABLE 4.
Predictors of becoming blood donor among non‐donors among the Danish National Health Survey participants.
| Baseline characteristic | HR (95% CI) crude | HR (95% CI) adjusted for sex and age | HR (95% CI) further adjusted |
|---|---|---|---|
| BMI: body mass index a | 0.92 (0.91–0.92) | 1.00 (0.99–1.01) | 1.01 (1.00–1.01) |
| PCS: SF‐12 physical component score a | 1.07 (1.07–1.07) | 1.03 (1.03–1.03) | 1.02 (1.02–1.03) |
| MCS: SF‐12 mental component score a | 0.99 (0.99–1.00) | 1.01 (1.01–1.01) | 1.01 (1.00–1.01) |
| BMI < 18.5 (thin) | 1.39 (1.23–1.56) | 0.68 (0.61–0.77) | 0.73 (0.65–0.82) |
| BMI 18.5 ≤ BMI < 25 (normal) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BMI 25 ≤ BMI < 30 (overweight) | 0.60 (0.57–0.64) | 1.08 (1.02–1.14) | 1.14 (1.07–1.21) |
| BMI 30 ≤ BMI (obese) | 0.42 (0.38–0.46) | 0.77 (0.70–0.85) | 0.89 (0.81–0.98) |
| PCS < 40 | 0.29 (0.25–0.34) | 0.51 (0.44–0.59) | 0.60 (0.51–0.69) |
| PCS 40 ≤ PCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| PCS 60 ≤ PCS | 1.78 (1.67–1.90) | 1.12 (1.05–1.20) | 1.08 (1.01–1.16) |
| MCS < 40 | 0.92 (0.86–0.99) | 0.74 (0.69–0.80) | 0.81 (0.76–0.87) |
| MCS 40 ≤ MCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| MCS 60 ≤ MCS | 0.56 (0.50–0.62) | 0.88 (0.79–0.98) | 0.89 (0.80–0.99) |
| Alcohol consumption: high risk | 1.46 (1.35–1.57) | 0.93 (0.86–1.00) | 0.94 (0.87–1.01) |
| Alcohol consumption: moderate | 1.44 (1.36–1.52) | 1.10 (1.04–1.17) | 1.08 (1.02–1.15) |
| Alcohol consumption: low risk | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Binge drinking: yes | 2.17 (2.07–2.28) | 1.20 (1.14–1.26) | 1.17 (1.11–1.23) |
| Binge drinking: no | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Smoking: current | 0.59 (0.56–0.63) | 0.68 (0.64–0.72) | 0.70 (0.66–0.75) |
| Smoking: former | 0.48 (0.45–0.52) | 1.01 (0.95–1.08) | 1.02 (0.96–1.10) |
| Smoking: never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: extreme | 3.10 (2.88–3.34) | 1.16 (1.07–1.25) | 1.14 (1.05–1.23) |
| Physical activity: high | 1.67 (1.58–1.76) | 1.33 (1.26–1.40) | 1.30 (1.23–1.38) |
| Physical activity: light | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: passive | 0.89 (0.81–0.97) | 0.74 (0.67–0.81) | 0.76 (0.69–0.83) |
Note: The hazard ratios (HR) denote increased (HR > 1) or decreased (HR < 1) rate of becoming a blood donor. The reference range for both the SF‐12 physical component score (PCS) and SF‐12 mental component score (MCS) was 0–100. Higher scores indicate better physical health for PCS and better mental health for MCS. Further adjusted HRs were adjusted for region (of residence at time of invitation), attained highest education (short (basic school), medium, long (12+ years)), and income (percentile of personal annual income). In addition, the predictors PCS, MCS, and BMI were also adjusted for smoking status, diet, alcohol consumption, and physical activity.
HRs per unit increase in score or value.
TABLE 5.
Predictors of lapsing for current donors among the Danish National Health Survey participants
| Baseline characteristic | HR (95% CI) crude | HR (95% CI) adjusted for sex and age | HR (95% CI) further adjusted |
|---|---|---|---|
| BMI: body mass index a | 0.99 (0.99–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) |
| PCS: SF‐12 physical component score a | 0.99 (0.99–0.99) | 0.99 (0.98–0.99) | 0.99 (0.98–0.99) |
| MCS: SF‐12 mental component score a | 0.98 (0.98–0.99) | 0.99 (0.99–0.99) | 0.99 (0.99–0.99) |
| BMI < 18.5 (thin) | 1.40 (1.15–1.71) | 1.21 (0.99–1.48) | 1.20 (0.98–1.47) |
| BMI 18.5 ≤ BMI < 25 (normal) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BMI 25 ≤ BMI < 30 (overweight) | 0.88 (0.85–0.92) | 0.98 (0.94–1.02) | 0.98 (0.94–1.02) |
| BMI 30 ≤ BMI (obese) | 1.01 (0.95–1.07) | 1.06 (0.99–1.12) | 1.05 (0.99–1.12) |
| PCS < 40 | 1.38 (1.25–1.52) | 1.45 (1.32–1.60) | 1.40 (1.27–1.55) |
| PCS 40 ≤ PCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| PCS 60 ≤ PCS | 1.17 (1.10–1.24) | 1.05 (0.99–1.12) | 1.04 (0.98–1.10) |
| MCS < 40 | 1.37 (1.29–1.46) | 1.28 (1.20–1.36) | 1.24 (1.16–1.32) |
| MCS 40 ≤ MCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| MCS 60 ≤ MCS | 0.85 (0.80–0.90) | 0.95 (0.89–1.01) | 0.96 (0.90–1.02) |
| Alcohol consumption: high risk | 1.14 (1.07–1.23) | 1.09 (1.02–1.17) | 1.07 (1.00–1.15) |
| Alcohol consumption: moderate | 1.05 (1.00–1.10) | 0.99 (0.95–1.04) | 0.98 (0.93–1.03) |
| Alcohol consumption: low risk | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Binge drinking: yes | 1.05 (1.01–1.09) | 1.01 (0.97–1.05) | 1.00 (0.96–1.04) |
| Binge drinking: no | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Smoking: current | 1.20 (1.14–1.26) | 1.21 (1.15–1.28) | 1.23 (1.17–1.29) |
| Smoking: former | 1.04 (0.99–1.09) | 1.12 (1.07–1.17) | 1.12 (1.07–1.17) |
| Smoking: never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: extreme | 1.09 (1.01–1.18) | 1.01 (0.93–1.10) | 1.00 (0.92–1.09) |
| Physical activity: high | 0.92 (0.89–0.96) | 0.93 (0.89–0.97) | 0.92 (0.89–0.96) |
| Physical activity: light | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: passive | 1.14 (1.07–1.23) | 1.13 (1.06–1.22) | 1.14 (1.06–1.23) |
Note: The hazard ratios (HR) denote increased (HR > 1) or decreased (HR < 1) rate of lapsing. The reference range for both the SF‐12 physical component score (PCS) and SF‐12 mental component score (MCS) is 0–100. Higher scores indicates better physical health for PCS and better mental health for MCS. Further adjusted HRs were adjusted for region (of residence at time of invitation), attained highest education (short(basic school), medium, long(12+ years)), and income (percentile of personal annual income). In addition, the predictors PCS, MCS, and BMI were also adjusted for smoking status, diet, alcohol consumption, and physical activity.
HRs per unit increase in score or value.
3.4. Predictors of blood donation intensity
Predictors of intensity of blood donation are presented in Table 6. The effect sizes were generally smaller than for previous outcomes. However, they seem more consistent, for example, all manner of not drinking alcohol and not smoking was associated with higher donation intensity. Being overweight (HR = 1.06 [1.04–1.07]) or obese (HR = 1.08 [1.06–1.10]) seemed most predictive of donation intensity.
TABLE 6.
Predictors of donation intensity for active donors among the Danish National Health Survey participants
| Baseline characteristic | HR (95% CI) crude | HR (95% CI) adjusted for sex and age | HR (95% CI) further adjusted |
|---|---|---|---|
| BMI: body mass index a | 1.01 (1.01–1.01) | 1.01 (1.01–1.01) | 1.01 (1.01–1.01) |
| PCS: SF‐12 physical component score a | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| MCS: SF‐12 mental component score a | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| BMI < 18.5 (thin) | 0.90 (0.83–0.98) | 0.98 (0.90–1.06) | 0.96 (0.88–1.05) |
| BMI 18.5 ≤ BMI < 25 (normal) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| BMI 25 ≤ BMI < 30 (overweight) | 1.10 (1.09–1.12) | 1.05 (1.04–1.06) | 1.06 (1.04–1.07) |
| BMI 30 ≤ BMI (obese) | 1.09 (1.07–1.11) | 1.07 (1.05–1.09) | 1.08 (1.06–1.10) |
| PCS < 40 | 1.06 (1.02–1.10) | 1.03 (1.00–1.07) | 1.01 (0.98–1.05) |
| PCS 40 ≤ PCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| PCS 60 ≤ PCS | 0.95 (0.94–0.97) | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) |
| MCS < 40 | 0.96 (0.94–0.98) | 1.00 (0.97–1.02) | 0.99 (0.96–1.01) |
| MCS 40 ≤ MCS < 60 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| MCS 60 ≤ MCS | 1.06 (1.04–1.08) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Alcohol consumption: high risk | 1.00 (0.98–1.02) | 1.02 (1.00–1.04) | 0.99 (0.97–1.01) |
| Alcohol consumption: moderate | 0.97 (0.95–0.98) | 1.00 (0.98–1.01) | 0.99 (0.98–1.00) |
| Alcohol consumption: low risk | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Binge drinking: yes | 0.97 (0.96–0.99) | 0.98 (0.97–0.99) | 0.98 (0.96–0.99) |
| Binge drinking: no | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Smoking: current | 0.99 (0.98–1.01) | 0.99 (0.97–1.00) | 0.97 (0.95–0.98) |
| Smoking: former | 1.00 (0.99–1.01) | 0.97 (0.96–0.99) | 0.96 (0.95–0.97) |
| Smoking: never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: extreme | 0.98 (0.96–1.01) | 1.01 (0.98–1.03) | 1.00 (0.98–1.03) |
| Physical activity: high | 1.01 (1.00–1.02) | 1.00 (0.98–1.01) | 1.00 (0.99–1.01) |
| Physical activity: light | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Physical activity: passive | 0.99 (0.96–1.01) | 0.99 (0.96–1.01) | 0.99 (0.97–1.01) |
Note: The hazard ratios (HR) denote increased (HR > 1) or decreased (HR < 1) rate of lapsing. The reference range for both the SF‐12 physical component score (PCS) and SF‐12 mental component score (MCS) is 0–100. Higher scores indicates better physical health for PCS and better mental health for MCS. Further adjusted HRs were adjusted for region (of residence at time of invitation), attained highest education (short(basic school), medium, long(12+ years)), and income (percentile of personal annual income). In addition, the predictors PCS, MCS, and BMI were also adjusted for smoking status, diet, alcohol consumption, and physical activity.
HRs per unit increase in score or value.
3.5. Mortality
Modeling the death rate among participants and non‐respondents at age <67 years after invitation based on age, sex, calendar year, participation, and donor status at time of invitation in a Poisson regression model, that is, a multiplicative model, we found a lower mortality in participants (HR = 0.51 [049–0.53]), active donors (HR = 0.40 [0.35–0.45]), and lapsed donors (HR = 0.83 [0.76–0.89]). This model implies that participation is associated with lower mortality in every stratum of donor behavior, for example, a participating active donor would have a HR of about 1/5 that of a non‐participating non‐donor. To back up this model claim we also produced similar models stratified by donor status and adjusted for sex and age and found participation to be associated with lower mortality in all donor strata; HR = 0.44 [0.33–0.59] (active donors) HR = 0.58 [0.48–0.65] (lapsed donors) and HR = 0.51 [0.49–0.53] (non‐donors).
4. DISCUSSION
We linked data on nearly 600,000 individuals invited to nationwide health surveys with a national database of Danish blood donors to explore the associations between blood donation career‐trajectories and participation rates, self‐reported health, modifiable lifestyles, and mortality, respectively. The underlying objective was to further characterize the HDE.
Low participation rates are a common characteristic of modern health surveys. Since participants typically are healthier than non‐participants, the resulting bias may compromise the ability to generalize survey observations to entire/similar populations. 21 , 22 The markedly lower mortality among participants compared with non‐participants (adjusted analyses) in this investigation was expected. 21 , 22 , 23 Our analyses demonstrated that history of blood donation was strongly associated with survey participation. This pertained to active and lapsed donors across all age groups, including above the upper age limit for blood donation (67 years at time of invitation). Participation was also correlated with number of recorded donations at time of invitation to the survey both in active and lapsed donors. For the active donors, these observations are similar to what was reported in the Dutch InSight investigation. 7 The overrepresentation of blood donors among study participants is perhaps unsurprising given the presumed underlying association between good health and survey participation. However, selection for good health may not suffice to explain the enrichment of survey participants by blood donors entirely.
Blood donors are routinely questioned about their health in relation to blood donation, and this may have contributed to their higher participation rates. Still, this would not explain why participation was also associated with future entry into the blood donor population. Thus, the increased participation rates across all donation career states may reflect that the same mechanisms that drive individuals to volunteer for blood donation also contribute to their willingness to participate in health surveys. This would not be without precedence; rather it would equate blood donors' proclivity to participate in health surveys with their increased willingness to also donate financially to charities. 24 , 25 , 26
The HDE was also manifested among survey participants. Mortality differed between participating current, lapsed, and non‐donors; active blood donors also reported better mental and physical health than lapsed and non‐blood donors. The observation of active blood donors' superior self‐reported health is in agreement with previous investigations, regardless of whether these have assessed this with a single question 1 , 7 , 9 or similar to the present investigation by separate scores for mental and physical health. 2 , 11 Among active donors, self‐reported mental health increased with number of donations while self‐reported physical health did not. This, too, is broadly consistent with observations in the Dutch InSight study. 7
Because of the cross‐sectional nature of our analyses, they do not directly inform about the mechanisms resulting in correlation between self‐reported health and donation activity. Still, by following study participants prospectively we found that better self‐reported physical and mental health both predicted becoming a blood donor, whereas poor self‐reported physical and mental health predicted donor lapse. Although the effect estimates were modest, these opposite selection phenomena indicate a continuous selection for increasingly good self‐reported health with increasing number of donations, especially for mental health. We found no association between self‐reported health and future donation intensity; however, our study may have been inadequately powered to this end. A recent study among participants in the Danish Blood Donor Study demonstrated modest associations between self‐reported mental health, and in female donors also self‐reported physical health, and future donation activity. 11 Likewise, self‐reported health correlated with donation activity in the Dutch Insight study. 9 Results of the Dutch Insight Study indicated that the strongest selection of blood donors for good health occur at enrolment into the blood donor population. That study also showed that, incipient, disease is an important risk factor for donor lapse. 7 , 9
We also investigated several lifestyle factors possibly associated with becoming, being, and lapsing as a blood donor. Donors differed from non‐donors on nearly all the examined parameters to some extent mirroring and possibly partially explaining their better self‐perceived health. The presented results were consistent with continuous selection of increasingly healthy individuals to become and remain active blood donors. Active donors were less likely to be smokers than non‐donors, and the prevalence of current smoking decreased with increasing number of donations. Current smoking was also associated with a lower likelihood of becoming a donor and with increased likelihood of lapsing, as well as lower donation intensity among active blood donors. Similar patterns were observed for the other explored characteristics. Relatedly, the observed results for binge drinking and extreme physical activity in relation to becoming and lapsing could indicate that the donor cohort features an influx and subsequent lapse of individuals with high‐experience acquisition lifestyles. However, confirmation of this speculation require additional analyses and data on characteristics pertaining to this personality type. Finally, the associations between overweight/obese BMI and continued donation was conceivably due to increased blood volumes in these individuals.
Our findings align with previous studies of the HDE. 1 , 2 , 7 , 9 , 11 , 27 Our results suggest that in addition to any theoretical beneficial effect repeated blood donations may have on donor health, the correlation between donor health and number of blood donations also reflects selection of healthy individuals to become blood donors and continuous selection of increasingly healthy individuals to remain active blood donors.
We provide evidence to suggest that the HDE could be expanded to include willingness to participate in health surveys. Irrespective of which mechanism might explain the overrepresentation of blood donors among health survey participants, our data show that participating donors are among the healthiest of donors with an extraordinary low mortality. Blood donors' willingness to participate in health surveys is of a sufficient scale that it is worth considering for these types of studies. This can partially be remedied by weighting the results according to response rates by sex, age, ethnicity, etc. We also show that within the donor population self‐reported health increases with donor career duration. We show that donor influx and efflux are associated with essentially opposite characteristics. Our results and other investigations suggest that the inclination to become blood donor is associated with good self‐reported health and health conscientious behavior. Independent of each other, these characteristics increase the ability to sustain potential adverse health effects that repeated blood donations entail, that is, constitute a healthy donor career / survivor effect. 7 , 9 Such a model would explain the correlation for self‐reported physical and mental health with number of donations among lapsed donors (J‐shaped dose–response pattern, data not shown).
The present investigation stands out from previous studies of the HDE in several ways. Firstly, the study population was surveyed independently and without reference to blood donor status. This limits the bias that might conceivably arise when health information is gathered in the setting of blood donation and/or compared with information for non‐donors collected in other settings. 2 , 7 Secondly, we could assess self‐perceived health and lifestyle both as predictor of becoming a blood donor and lapsing using accurate information on blood donation from SCANDAT. 11 , 14 , 18 , 28 Thirdly, information from health registers enabled examinations of mortality according to both donor career‐ and health‐survey status.
We consider the internal validity of this study to be high. The outcomes, and most predictors in the study period (death, donation histories, sex, birthdate, calendar time, income, place of residence, education), were objective facts registered with almost 100% completeness and accuracy. 11 , 14 , 18 , 27 , 28 The self‐reported survey questions used are mostly well‐validated workhorses of epidemiology and survey sampling. 17 , 29 , 30 , 31 , 32 , 33 One exception was the item on binge drinking, as the wording/definition was changed between surveys, >5 × 12 g alcohol on a single occasion in 2010 but ≥5 × 12 g in 2013. The survey participants had no incentive to be dishonest and questions were not complicated to answer. 22 The statistical models used are definitely too simplistic as tools for prediction, but for this broad‐brush description of assumed causal associations we consider them adequate. 34
As demonstrated, a study base of blood donors differs from the background population in important ways, which must be accounted for in related studies. Conversely, the relative homogeneity of the donor population is actually a blessing, to promote internal validity and reliable inference, that is generalizable exactly because it is an expression of a pathophysiological mechanism. This would seem especially to be the case for studying relations between sub‐clinical biomarkers of pathophysiology in the healthy human host, that is, phenomena you would expect not to depend on personality, education, income, and ethnicity. 35 , 36 , 37 , 38
In conclusion, we provide evidence that history of blood donation is associated with an increased likelihood to participate in public health surveys, and that this may be a manifestation of the healthy donor or the healthy donor survivor effect. We also demonstrate that correlates of future blood donation choices—becoming or lapsing—would account for increasingly good self‐reported health and increasingly healthy lifestyles with increasing number of donations.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Figure S1. Cohort diagram. The study population comprised of data from the Danish National Health Survey (DNHS) 2010 and 2013. The data were combined to form a single study population. For individuals invited to both the 2010 and 2013 survey, only data for 2010 was retained. Using unique personal identification numbers issued to all individuals living in Denmark, data from DNHS 2010 and 2013 was linked to SCANDAT to obtain information on donation history and to Danish registers to obtain information on vital status and socio‐demographic characteristics. DNHS participants and non‐respondents were categorized into three donor strata: Active, lapsed, and non‐donors. Individuals with non‐complete survey data or scoring outside the reference ranges of relevant predictors were excluded from the analyses.
ACKNOWLEDGMENTS
This study was supported by a grant from Helsefonden (grant‐number 21‐B‐0432 to HHJ).
Brodersen T, Rostgaard K, Lau CJ, Juel K, Erikstrup C, Nielsen KR, et al. The healthy donor effect and survey participation, becoming a donor and donor career. Transfusion. 2023;63(1):143–155. 10.1111/trf.17190
Funding information Helsefonden, Grant/Award Number: 21‐B‐0432 to HHJ
REFERENCES
- 1. Shehu E, Hofmann A, Clement M, et al. Healthy donor effect and satisfaction with health: the role of selection effects related to blood donation behavior. Eur J Heal Econ. 2015;16(7):733–45. 10.1007/s10198-014-0625-1 [DOI] [PubMed] [Google Scholar]
- 2. Rigas AS, Skytthe A, Erikstrup C, et al. The healthy donor effect impacts self‐reported physical and mental health – results from the Danish Blood Donor Study (DBDS). Transfus Med. 2017;29(S1):65–9. 10.1111/tme.12478 [DOI] [PubMed] [Google Scholar]
- 3. Edgren G, Tran TN, Hjalgrim H, et al. Improving health profile of blood donors as a consequence of transfusion safety efforts. Transfusion. 2007;47(11):2017–24. 10.1111/j.1537-2995.2007.01425.x [DOI] [PubMed] [Google Scholar]
- 4. Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion. 2002;42(9):1135–9. 10.1046/j.1537-2995.2002.00186.x [DOI] [PubMed] [Google Scholar]
- 5. Tuomainen T‐P, Salonen R, Nyyssonen K, et al. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793–3. 10.1136/bmj.314.7083.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol. 2005;25(8):1577–83. 10.1161/01.ATV.0000174126.28201.61 [DOI] [PubMed] [Google Scholar]
- 7. Atsma F, Veldhuizen I, Verbeek A, et al. Healthy donor effect: its magnitude in health research among blood donors. Transfusion. 2011;51(8):1820–8. 10.1111/j.1537-2995.2010.03055.x [DOI] [PubMed] [Google Scholar]
- 8. Atsma F, de Vegt F. The healthy donor effect: a matter of selection bias and confounding. Transfusion. 2011;51(9):1883–5. 10.1111/j.1537-2995.2011.03270.x [DOI] [PubMed] [Google Scholar]
- 9. Van Den Hurk K, Zalpuri S, Prinsze FJ, et al. Associations of health status with subsequent blood donor behavior — an alternative perspective on the healthy donor effect from donor inSight. PLoS One. 2017;12(10):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ullum H, Rostgaard K, Kamper‐Jørgensen M, et al. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion. 2015. Oct;55(10):2479–85. 10.1111/trf.13205 [DOI] [PubMed] [Google Scholar]
- 11. Didriksen M, Thørner LW, Larsen MAH, et al. The impact of health‐related quality of life and depressive symptoms on blood donor career ‐ results from the Danish Blood Donor Study. Transfusion. 2021;61(5):1479–88. 10.1111/trf.16336 [DOI] [PubMed] [Google Scholar]
- 12. Gallerani M, Volpato S, Cellini M, et al. Risk of illness, hospitalization and death in a cohort of blood donors in Italy. Curr Med Res Opin. 2014;30(9):1803–12. 10.1185/03007995.2014.921146 [DOI] [PubMed] [Google Scholar]
- 13. Christensen AI, Ekholm O, Glümer C, et al. The Danish National Health Survey 2010. Study design and respondent characteristics. Scand J Public Health. 2012;40(4):391–7. 10.1177/1403494812451412 [DOI] [PubMed] [Google Scholar]
- 14. Edgren G, Rostgaard K, Vasan SK, et al. The new Scandinavian donations and transfusions database (SCANDAT2): a blood safety resource with added versatility. Transfusion. 2015. Jul;55(7):1600–6. 10.1111/trf.12986 [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB. The Danish civil registration system. Scand J Public Health [Internet]. 2011;39(7_suppl):22–5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 16. Christensen AI, Lau CJ, Kristensen PL, et al. The Danish National Health Survey: study design, response rate and respondent characteristics in 2010, 2013 and 2017. Scand J Public Health. 2020;50(2):180–8. 10.1177/1403494820966534 [DOI] [PubMed] [Google Scholar]
- 17. Ware JE, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. Available from: http://journals.lww.com/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 18. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 19. Ware J, Kosinski M, Keller S. SF‐12: how to score the SF‐12 physical and mental health summary scales. 2nd ed. Boston, MA: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 20. R Core Team . R: a language and environment for statistical computing [internet]. Vienna, Austria; 2020. Available from: https://www.r-project.org/ [Google Scholar]
- 21. Lallukka T, Pietiläinen O, Jäppinen S, Laaksonen M, Lahti J, Rahkonen O. Factors associated with health survey response among young employees: a register‐based study using online, mailed and telephone interview data collection methods. BMC Public Health. 2020;20(1):184. 10.1186/s12889-020-8241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jensen HAR, Ekholm O, Davidsen M, et al. The Danish health and morbidity surveys: study design and participant characteristics. BMC Med Res Methodol. 2019;19(1):91. 10.1186/s12874-019-0733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keyes KM, Rutherford C, Popham F, Martins SS, Gray L. How healthy are survey respondents compared with the general population? Epidemiology. 2018;29(2):299–307. Available from: http://journals.lww.com/00001648-201803000-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee L, Piliavin JA, Call VRA. Giving time, money, and blood: similarities and differences. Soc Psychol Q. 1999;62(3):276 Available from: http://www.jstor.org/stable/2695864?origin=crossref [Google Scholar]
- 25. Chell K, Davison TE, Masser B, Jensen K. A systematic review of incentives in blood donation. Transfusion. 2018;58(1):242–54. 10.1111/trf.14387 [DOI] [PubMed] [Google Scholar]
- 26. Ferguson E, Murray C, O'Carroll RE. Blood and organ donation: health impact, prevalence, correlates, and interventions. Psychol Health. 2019;34(9):1073–104. 10.1080/08870446.2019.1603385 [DOI] [PubMed] [Google Scholar]
- 27. Burgdorf KS, Simonsen J, Sundby A, et al. Socio‐demographic characteristics of Danish Blood Donors. PLoS One. 2017;12(2):e0169112. 10.1371/journal.pone.0169112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao J, Rostgaard K, Hjalgrim H, Edgren G. The Swedish Scandinavian donations and transfusions database (SCANDAT3‐S) – 50 years of donor and recipient follow‐up. Transfusion. 2020;60(12):3019–27. 10.1111/trf.16027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steenstrup T, Pedersen OB, Hjelmborg J, et al. Heritability of health‐related quality of life: SF‐12 summary scores in a population‐based nationwide twin cohort. Twin Res Hum Genet. 2013;16(3):670–8. Available from: https://www.cambridge.org/core/product/identifier/S1832427413000212/type/journal_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gandek B, Ware JE, Aaronson NK, et al. Cross‐validation of item selection and scoring for the SF‐12 health survey in nine countries. J Clin Epidemiol. 1998;51(11):1171–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0895435698001097 [DOI] [PubMed] [Google Scholar]
- 31. Steffensen HH, Lauritzen T, Sørensen HT. Validity of self‐reported smoking habits. Scand J Prim Health Care. 1995;13(3):236–7. 10.3109/02813439508996767 [DOI] [PubMed] [Google Scholar]
- 32. Zierau F, Hardt F, Henriksen JH, et al. Validation of a self‐administered modified CAGE test (CAGE‐C) in a somatic hospital ward: comparison with biochemical markers. Scand J Clin Lab Invest. 2005;65(7):615–22. 10.1080/00365510500333445 [DOI] [PubMed] [Google Scholar]
- 33. Neermark S, Holst C, Bisgaard T, et al. Validation and calibration of self‐reported height and weight in the Danish health examination survey. Eur J Public Health. 2019;29(2):291–6. Available from: https://academic.oup.com/eurpub/article/29/2/291/5100738 [DOI] [PubMed] [Google Scholar]
- 34. Arnold KF, Davies V, de Kamps M, et al. Reflection on modern methods: generalized linear models for prognosis and intervention—theory, practice and implications for machine learning. Int J Epidemiol. 2020;49(6):2074–82. Available from: https://academic.oup.com/ije/article/49/6/2074/5831974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. von Stemann JH, Rigas AS, Thørner LW, et al. Prevalence and correlation of cytokine‐specific autoantibodies with epidemiological factors and C‐reactive protein in 8,972 healthy individuals: results from the Danish Blood Donor Study. PLoS One. 2017;12(6):e0179981. 10.1371/journal.pone.0179981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotzé SR, Pedersen OB, Petersen MS, et al. Low‐grade inflammation is associated with lower haemoglobin levels in healthy individuals: results from the Danish blood donor study. Vox Sang. 2016. Aug;111(2):144–50. 10.1111/vox.12396 [DOI] [PubMed] [Google Scholar]
- 37. Kaspersen KA, Pedersen OB, Petersen MS, et al. Obesity and risk of infection. Epidemiology. 2015;26(4):580–9. Available from: http://journals.lww.com/00001648-201507000-00019 [DOI] [PubMed] [Google Scholar]
- 38. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low‐grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220. 10.1371/journal.pone.0164220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cohort diagram. The study population comprised of data from the Danish National Health Survey (DNHS) 2010 and 2013. The data were combined to form a single study population. For individuals invited to both the 2010 and 2013 survey, only data for 2010 was retained. Using unique personal identification numbers issued to all individuals living in Denmark, data from DNHS 2010 and 2013 was linked to SCANDAT to obtain information on donation history and to Danish registers to obtain information on vital status and socio‐demographic characteristics. DNHS participants and non‐respondents were categorized into three donor strata: Active, lapsed, and non‐donors. Individuals with non‐complete survey data or scoring outside the reference ranges of relevant predictors were excluded from the analyses.


