FIGURE 1.
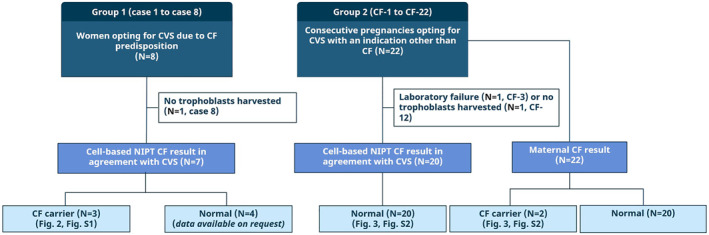
The flow of patients and samples. Blood was collected from two groups of pregnant women receiving invasive sampling. In group 1, the pregnancies were included when they opted for invasive sampling for Cystic fibrosis (CF) due to the couple's carrier status or family history. This group was the validation group. In group 2, N = 22 pregnant women were included consecutively when they opted for invasive sampling with an indication other than CF. This group simulated a prenatal screening program with a CF risk corresponding to the background population. The text describes the outcome for cell‐based noninvasive prenatal test (NIPT) for both groups as well as the maternal CF result in group 2. Abbreviations: CF, cystic fibrosis; CVS, chorionic villous sampling.
