FIGURE 3.
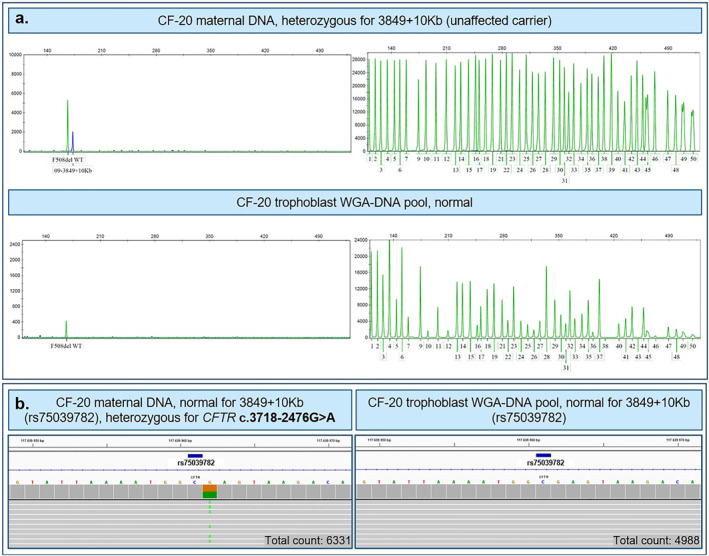
CF test result for maternal DNA and trophoblast WGA‐DNA pools for CF‐20 using (A) Amplification Refractory Mutation System polymerase chain reaction (ARMS‐PCR) and fragment length analysis and (B) next‐generation‐sequencing. (A) The maternal Cystic fibrosis (CF) test result presents with a blue variant peak 09–3849 + 10Kb indicating that the pregnant woman is a heterozygote carrier of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) 3849 + 10kbC > T (c.3718‐2477C > T, dbSNP: rs75039782). The trophoblast WGA‐DNA showed a normal CF test result, indicating that the fetus is unaffected. Panel (B) shows the sequencing analysis of the variant, rs75039782, and the base position is marked in dark blue bar. The grey bars represent sequences that do not vary from the hg38 reference genome, displayed by the colored base sequence. In the maternal test result, an single nucleotide variant (SNV), CFTR c.3718‐2476 G > A, was detected 1 bp downstream for the variant of interest. This SNV is a likely benign intron variant. Thus, the ARMS‐PCR result was due to an SNV in the primer binding site, resulting in a false‐positive result. The CF‐20 trophoblast WGA‐DNA sequencing result presented with a normal CF test result. Total counts for the specific amplicon are specified in the lower right corner. Abbreviations: ARMS‐PCR, Amplification Refractory Mutation System polymerase chain reaction; bp, base pair; CF, cystic fibrosis; SNV, single nucleotide variant; WGA‐DNA, whole genome amplified DNA.
